Intergenerational effects of macroalgae on a reef coral: major declines in larval survival but subtle changes in microbiomes
- PMID: 30505048
- PMCID: PMC6261492
- DOI: 10.3354/meps12465
Intergenerational effects of macroalgae on a reef coral: major declines in larval survival but subtle changes in microbiomes
Abstract
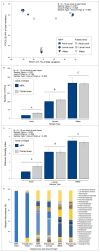
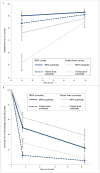
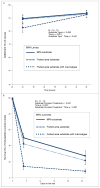
Tropical reefs are shifting from coral to macroalgal dominance, with macroalgae suppressing coral recovery, potentially via effects on coral microbiomes. Understanding how macroalgae affect corals and their microbiomes requires comparing algae- versus coral-dominated reefs without confounding aspects of time and geography. We compared survival, settlement, and post-settlement survival of larvae, as well as the microbiomes of larvae and adults, of the Pacific coral Pocillopora damicornis between an Marine Protected Area (MPA) dominated by corals versus an adjacent fished area dominated by macroalgae. Microbiome composition in adult coral, larval coral, and seawater did not differ between the MPA and fished area. However, microbiomes of adult coral were more variable in the fished area and Vibrionaceae bacteria, including strains most closely related to the pathogen Vibrio shilonii, were significantly enriched, but rare, in adult and larval coral from the fished area. Larvae from the macroalgae-dominated area exhibited higher pre-settlement mortality and reduced settlement compared to those from the coral-dominated area. Juveniles planted into a coral-dominated area survived better than those placed into a fished area dominated by macroalgae. Differential survival depended on whether macroalgae were immediately adjacent to juvenile coral rather than on traits of the areas per se. Contrary to our expectations, coral microbiomes were relatively uniform at the community level despite dramatic differences in macroalgal cover between the MPA (~2% cover) and fished (~90%) area. Reducing macroalgae may elicit declines in rare but potentially harmful microbes in coral and their larvae, as well as positive intergenerational effects on offspring survival.
Keywords: Pocillopora damicornis; coral larvae; coral microbiome; coral-algae interactions; marine protected areas.
Figures


References
-
- Ainsworth TD, Thurber RV, Gates RD. The future of coral reefs: a microbial perspective. Trends Ecol Evol. 2010;25:233–240. - PubMed
-
- Almany GR, Connolly SR, Heath DD, Hogan JD, Jones GP, McCook LJ, Mills M, Pressey RL, Williamson DH. Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs. 2009;28:339–351.
-
- Barott KL, Rohwer FL. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 2012;20:621–628. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
