Efficacy of rebamipide 2% ophthalmic solution in the treatment of dry eyes
- PMID: 30505109
- PMCID: PMC6219330
- DOI: 10.4103/ojo.OJO_29_2017
Efficacy of rebamipide 2% ophthalmic solution in the treatment of dry eyes
Abstract
Aim: The aim of this study is to evaluate the efficacy of 2% rebamipide ophthalmic solution on the tear functions and ocular surface status in patients with dry eyes.
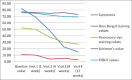
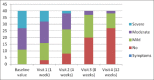
Materials and methods: A prospective study was carried out on forty eyes of patients having signs and symptoms of dry eye. Two percent rebamipide ophthalmic solution was applied four times a day for a period of 4 weeks. Patients were evaluated on the basis of dry eye-related symptom score, tear film break-up time (TFBUT), tear meniscus height, fluorescein ocular surface staining score (FOSS), and the Schirmer's test at the end of 2, 4, 6, and 12 weeks and their values were compared with the baseline.
Results: Significant improvement was noted in the mean dry eye-related symptom score, TBUT, and the FOSS values from the baseline at the end of 2, 4, 8, and 12 weeks. The values of tear meniscus height and Schirmer's test were improved at the end of 8 weeks but were not statistically significant.
Conclusion: Nearly 2% rebamipide ophthalmic solution provided relief in the symptoms of patients having dry eyes. It also prevented further ocular surface damage and aided in stabilizing the tear film.
Keywords: Dry eye; mucin secretagogue; rebamipide.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Sharma B. Dry eye: Demography and attributable risk factors. Postgrad Med J NAMS. 2011;11:20–1.
-
- Lemp MA. Management of the dry-eye patient. Int Ophthalmol Clin. 1994;34:101–13. - PubMed
-
- Tsubota K. New approaches to dry-eye therapy. Int Ophthalmol Clin. 1994;34:115–28. - PubMed
-
- Baldone JA, Kaufman HE. Extended wear contact lenses. Ann Ophthalmol. 1983;15:595–6. - PubMed