Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia
- PMID: 30505304
- PMCID: PMC6250824
- DOI: 10.3389/fimmu.2018.02640
Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia
Abstract
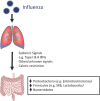
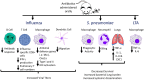
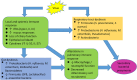
Influenza and other respiratory viral infections are the most common type of acute respiratory infection. Viral infections predispose patients to secondary bacterial infections, which often have a more severe clinical course. The mechanisms underlying post-viral bacterial infections are complex, and include multifactorial processes mediated by interactions between viruses, bacteria, and the host immune system. Studies over the past 15 years have demonstrated that unique microbial communities reside on the mucosal surfaces of the gastrointestinal tract and the respiratory tract, which have both direct and indirect effects on host defense against viral infections. In addition, antiviral immune responses induced by acute respiratory infections such as influenza are associated with changes in microbial composition and function ("dysbiosis") in the respiratory and gastrointestinal tract, which in turn may alter subsequent immune function against secondary bacterial infection or alter the dynamics of inter-microbial interactions, thereby enhancing the proliferation of potentially pathogenic bacterial species. In this review, we summarize the literature on the interactions between host microbial communities and host defense, and how influenza, and other acute respiratory viral infections disrupt these interactions, thereby contributing to the pathogenesis of secondary bacterial infections.
Keywords: bacterial pneumonia; gut microbiome; host-microbe interaction; influenza; respiratory viral infection; viral-bacterial interaction.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

