Glial responses to implanted electrodes in the brain
- PMID: 30505625
- PMCID: PMC6261524
- DOI: 10.1038/s41551-017-0154-1
Glial responses to implanted electrodes in the brain
Erratum in
-
Publisher Correction: Glial responses to implanted electrodes in the brain.Nat Biomed Eng. 2018 Jan;2(1):52. doi: 10.1038/s41551-017-0177-7. Nat Biomed Eng. 2018. PMID: 31015653
Abstract
The use of implants that can electrically stimulate or record electrophysiological or neurochemical activity in nervous tissue is rapidly expanding. Despite remarkable results in clinical studies and increasing market approvals, the mechanisms underlying the therapeutic effects of neuroprosthetic and neuromodulation devices, as well as their side effects and reasons for their failure, remain poorly understood. A major assumption has been that the signal-generating neurons are the only important target cells of neural-interface technologies. However, recent evidence indicates that the supporting glial cells remodel the structure and function of neuronal networks and are an effector of stimulation-based therapy. Here, we reframe the traditional view of glia as a passive barrier, and discuss their role as an active determinant of the outcomes of device implantation. We also discuss the implications that this has on the development of bioelectronic medical devices.
Conflict of interest statement
Competing interests The authors declare no competing financial interests.
Figures

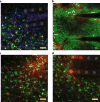




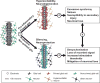
References
-
- Kubota Y. Untangling GABAergic wiring in the cortical microcircuit. Curr. Opin. Neurobiol. 2014;26:7–14. - PubMed
-
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. - PubMed
-
- Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–171. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

