Disordered Peptides Looking for Their Native Environment: Structural Basis of CB1 Endocannabinoid Receptor Binding to Pepcans
- PMID: 30505835
- PMCID: PMC6250848
- DOI: 10.3389/fmolb.2018.00100
Disordered Peptides Looking for Their Native Environment: Structural Basis of CB1 Endocannabinoid Receptor Binding to Pepcans
Abstract
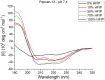
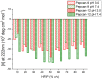
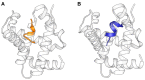
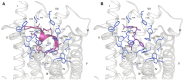
Endocannabinoid peptides, or "pepcans," are endogenous ligands of the CB1 cannabinoid receptor. Depending on their length, they display diverse activity: For instance, the nona-peptide Pepcan-9, also known as hemopressin, is a powerful inhibitor of CB1, whereas the longer variant Pepcan-12, which extends by only three amino acid residues at the N-terminus, acts on both CB1 and CB2 as an allosteric modulator, although with diverse effects. Despite active research on their pharmacological applications, very little is known about structure-activity relationships of pepcans. Different structures have been proposed for the nona-peptide, which has also been reported to form fibrillar aggregates. This might have affected the outcome and reproducibility of bioactivity studies. In an attempt of elucidating the determinants of both biological activity and aggregation propensity of Pepcan-9 and Pepcan-12, we have performed their structure characterization in solvent systems characterized by different polarity and pH. We have found that, while disordered in aqueous environment, both peptides display helical structure in less polar environment, mimicking the proteic receptor milieu. In the case of Pepcan-9, this structure is fully consistent with the observed modulation of the CB1. For Pepcan-12, whose allosteric binding site is still unknown, the presented structure is compatible with the binding at one of the previously proposed allosteric sites on CB1. These findings open the way to structure-driven design of selective peptide modulators of CB1.
Keywords: CB1 endocannabinoid receptor; endocannabinoid system; hemopressin; intrinsically unfolded peptides; pepcans; structure-activity relationships.
Figures

Similar articles
-
Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors.J Biol Chem. 2012 Oct 26;287(44):36944-67. doi: 10.1074/jbc.M112.382481. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952224 Free PMC article.
-
Characterization of pepcan-23 as pro-peptide of RVD-hemopressin (pepcan-12) and stability of hemopressins in mice.Adv Biol Regul. 2021 May;80:100808. doi: 10.1016/j.jbior.2021.100808. Epub 2021 Mar 24. Adv Biol Regul. 2021. PMID: 33799079
-
Pepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damage.Sci Rep. 2017 Aug 25;7(1):9560. doi: 10.1038/s41598-017-09808-8. Sci Rep. 2017. PMID: 28842619 Free PMC article.
-
Hemopressin Peptides as Modulators of the Endocannabinoid System and their Potential Applications as Therapeutic Tools.Protein Pept Lett. 2016;23(12):1045-1051. doi: 10.2174/0929866523666161007152435. Protein Pept Lett. 2016. PMID: 27748182 Review.
-
Endocannabinoids and Their Pharmacological Actions.Handb Exp Pharmacol. 2015;231:1-37. doi: 10.1007/978-3-319-20825-1_1. Handb Exp Pharmacol. 2015. PMID: 26408156 Review.
Cited by
-
Endocannabinoid signaling in the central nervous system.Glia. 2023 Jan;71(1):5-35. doi: 10.1002/glia.24280. Epub 2022 Oct 29. Glia. 2023. PMID: 36308424 Free PMC article.
-
Allosteric Modulator Leads Hiding in Plain Site: Developing Peptide and Peptidomimetics as GPCR Allosteric Modulators.Front Chem. 2021 Oct 7;9:671483. doi: 10.3389/fchem.2021.671483. eCollection 2021. Front Chem. 2021. PMID: 34692635 Free PMC article. Review.
-
Hemopressin as a breakthrough for the cannabinoid field.Neuropharmacology. 2021 Feb 1;183:108406. doi: 10.1016/j.neuropharm.2020.108406. Epub 2020 Nov 16. Neuropharmacology. 2021. PMID: 33212113 Free PMC article. Review.
-
How well does molecular simulation reproduce environment-specific conformations of the intrinsically disordered peptides PLP, TP2 and ONEG?Chem Sci. 2022 Jan 20;13(7):1957-1971. doi: 10.1039/d1sc03496k. eCollection 2022 Feb 16. Chem Sci. 2022. PMID: 35308859 Free PMC article.
-
The electrophysiological and behavioral evaluation of the peptide hemopressin and cannabinoid CB1 receptor agonist and antagonist in pentylenetetrazol model of epilepsy in rats.Pflugers Arch. 2023 Jun;475(6):719-730. doi: 10.1007/s00424-023-02814-y. Epub 2023 Apr 27. Pflugers Arch. 2023. PMID: 37100982
References
-
- Bauer M., Chicca A., Tamborrini M., Eisen D., Lerner R., Lutz B., et al. . (2012). Identification and quantification of a new family of peptide endocannabinoids (pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 287, 36944–36967. 10.1074/jbc.M112.382481 - DOI - PMC - PubMed
-
- Bernardi M. L., Picone D., Tuppo L., Giangrieco I., Petrella G., Palazzo P., et al. . (2010). Physico-chemical features of the environment affect the protein conformation and the immunoglobulin E reactivity of kiwellin (Act d 5). Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 40, 1819–1826. 10.1111/j.1365-2222.2010.03603.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

