Metatranscriptomic and Thermodynamic Insights into Medium-Chain Fatty Acid Production Using an Anaerobic Microbiome
- PMID: 30505946
- PMCID: PMC6247018
- DOI: 10.1128/mSystems.00221-18
Metatranscriptomic and Thermodynamic Insights into Medium-Chain Fatty Acid Production Using an Anaerobic Microbiome
Abstract
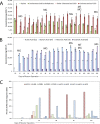
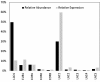
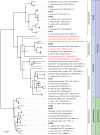
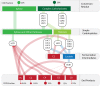
Biomanufacturing from renewable feedstocks can offset fossil fuel-based chemical production. One potential biomanufacturing strategy is production of medium-chain fatty acids (MCFA) from organic feedstocks using either pure cultures or microbiomes. While the set of microbes in a microbiome can often metabolize organic materials of greater diversity than a single species can and while the role of specific species may be known, knowledge of the carbon and energy flow within and between organisms in MCFA-producing microbiomes is only now starting to emerge. Here, we integrated metagenomic, metatranscriptomic, and thermodynamic analyses to predict and characterize the metabolic network of an anaerobic microbiome producing MCFA from organic matter derived from lignocellulosic ethanol fermentation conversion residue. A total of 37 high-quality (>80% complete, <10% contamination) metagenome-assembled genomes (MAGs) were recovered from the microbiome, and metabolic reconstruction of the 10 most abundant MAGs was performed. Metabolic reconstruction combined with metatranscriptomic analysis predicted that organisms affiliated with Lactobacillus and Coriobacteriaceae would degrade carbohydrates and ferment sugars to lactate and acetate. Lachnospiraceae- and Eubacteriaceae-affiliated organisms were predicted to transform these fermentation products to MCFA. Thermodynamic analyses identified conditions under which H2 is expected to be either produced or consumed, suggesting a potential role of H2 partial pressure in MCFA production. From an integrated systems analysis perspective, we propose that MCFA production could be improved if microbiomes were engineered to use homofermentative instead of heterofermentative Lactobacillus and if MCFA-producing organisms were engineered to preferentially use a thioesterase instead of a coenzyme A (CoA) transferase as the terminal enzyme in reverse β-oxidation. IMPORTANCE Mixed communities of microbes play important roles in health, the environment, agriculture, and biotechnology. While tapping the combined activities of organisms within microbiomes may allow the utilization of a wider range of substrates in preference to the use of pure cultures for biomanufacturing, harnessing the metabolism of these mixed cultures remains a major challenge. Here, we predicted metabolic functions of bacteria in a microbiome that produces medium-chain fatty acids from a renewable feedstock. Our findings lay the foundation for efforts to begin addressing how to engineer and control microbiomes for improved biomanufacturing, how to build synthetic mixtures of microbes that produce valuable chemicals from renewable resources, and how to better understand the microbial communities that contribute to health, agriculture, and the environment.
Keywords: anaerobic digestion; biorefining; carboxylate platform; hexanoic acid; medium-chain fatty acids; metagenomics; metatranscriptomics; octanoic acid.
Figures

References
-
- Angenent LT, Richter H, Buckel W, Spirito CM, Steinbusch KJJ, Plugge CM, Strik D, Grootscholten TIM, Buisman CJN, Hamelers HVM. 2016. Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environ Sci Technol 50:2796–2810. doi:10.1021/acs.est.5b04847. - DOI - PubMed
-
- Agler MT, Spirito CM, Usack JG, Werner JJ, Angenent LT. 2012. Chain elongation with reactor microbiomes: upgrading dilute ethanol to medium-chain carboxylates. Energy Environ Sci 5:8189. doi:10.1039/c2ee22101b. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
