Spectral-Domain OCT Findings of Retinal Vascular-Avascular Junction in Infants with Retinopathy of Prematurity
- PMID: 30506013
- PMCID: PMC6261282
- DOI: 10.1016/j.oret.2018.02.001
Spectral-Domain OCT Findings of Retinal Vascular-Avascular Junction in Infants with Retinopathy of Prematurity
Abstract
Purpose: Bedside examination of premature infants at risk for retinopathy of prematurity (ROP) is predominantly performed with ophthalmoscopic en face viewing of the retina. While postmortem retinal microstructures have been studied at the vascular-avascular junction, a critical location for pathogenesis of ROP, to date this has not been possible in vivo. Here we present bedside, non-sedated in vivo cross-sectional imaging and analysis of retinal microstructures at the vascular-avascular junction in infants with ROP using handheld spectral-domain optical coherence tomography (SDOCT).
Design: Prospective observational study.
Participants: Eleven preterm infants consented for research imaging during ROP screening examinations.
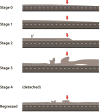
Methods: We imaged the vascular-avascular junction in the temporal retina using a SDOCT system (Envisu, Bioptigen Inc., NC) in 18 eyes from 11 preterm infants with zone I or II, stage 0 through 4 ROP. B-scan and en face images were analyzed and compared to historical light micrographs.
Main outcome measures: SDOCT morphology at the vascular-avascular junction.
Results: Multiple bedside SDOCT findings at the vascular-avascular junction were comparable to historic light micrographs: thickened inner retinal ridge structure in stage 2 ROP was comparable to thickened vanguard and rear guard cells in micrographs; vascular tufts on the posterior retinal surface in stage 2 ROP, broad arcs of neovascularization above the retina in stage 3 ROP, and splitting of inner retinal layers into clefts on either side of neovascularization mimicked findings of historic light micrographs. A unique findings was thickening of the avascular inner retinal band adjacent to neovascularization. On SDOCT imaging over several weeks, neovascularization and retinal clefts diminished after intravitreal bevacizumab therapy.
Conclusions: Retinal morphology at the vascular-avascular junction imaged with handheld SDOCT is consistent with known histopathology, and provide the advantage of monitoring change in vivo over time. These unique findings provide new insights into preterm retinal neurovascular development in ROP.
Conflict of interest statement
Conflict of Interest: No other authors have financial disclosures. No authors have a proprietary interest in the current study.
Figures




References
-
- Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98(11):1628–40. - PubMed
-
- Quinn GE, Dobson V, Barr CC, et al. Visual acuity in infants after vitrectomy for severe retinopathy of prematurity. Ophthalmology. 1991;98(1):5–13. - PubMed
-
- Tasman W. Retinopathy of prematurity: do we still have a problem?: the Charles L. Schepens lecture. Arch Ophthalmol. 2011;129(8):1083–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
