Radiolabeled Angiogenesis-Targeting Croconaine Nanoparticles for Trimodality Imaging Guided Photothermal Therapy of Glioma
- PMID: 30506043
- PMCID: PMC6261507
- DOI: 10.1021/acsanm.8b00195
Radiolabeled Angiogenesis-Targeting Croconaine Nanoparticles for Trimodality Imaging Guided Photothermal Therapy of Glioma
Abstract
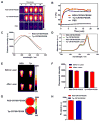
To meet the criteria of effective theranostics, biocompatible nanomedicine endowing intrinsic therapeutic and imaging properties have gained extraordinary momentum. In this study, an ultra-stable near-infrared (NIR) dye croconaine (CR780) was engineered with arginine-glycine-aspartic acid (RGD) peptide and polyethylene glycol (PEG), which was then self-assembled into uniform nanoparticles (NPs). These RGD-CR780-PEG5K assemblies were radiolabeled with 125I through a facile standard Iodo-Gen method. The resulting [125I]RGD-CR780-PEG5K NPs showed effective accumulation in αvβ3 integrin expressing glioblastoma, as evidenced by single photon emission computed tomography (SPECT)/CT and NIR fluorescence imaging. More importantly, high-resolution photoacoustic imaging revealed that these NPs selectively targeted to angiogenic tumor vessels. With the favorable tumor selective accumulation and high photothermal conversion efficiency, the [125I]RGD-CR780-PEG5K NPs allowed thorough tumor ablation and inhibition of tumor relapse at a relatively low laser energy (0.5 W/cm2). Overall, this work offers a proper methodology to fabricate tumor-targeted multi-modal nanotheranostic agents, providing great opportunity for precision imaging and cancer therapy.
Keywords: SPECT/CT; angiogenesis; croconaine nanoparticles; glioma; photothermal therapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Croconaine nanoparticles with enhanced tumor accumulation for multimodality cancer theranostics.Biomaterials. 2017 Jun;129:28-36. doi: 10.1016/j.biomaterials.2017.03.009. Epub 2017 Mar 13. Biomaterials. 2017. PMID: 28324863
-
Facile Synthesis of a Croconaine-Based Nanoformulation for Optoacoustic Imaging and Photothermal Therapy.Adv Healthc Mater. 2021 May;10(9):e2002115. doi: 10.1002/adhm.202002115. Epub 2021 Mar 18. Adv Healthc Mater. 2021. PMID: 33738974 Free PMC article.
-
131I-Labeled arginine-arginine-leucine (RRL)-containing cyclic peptide (YCGGRRLGGC) for imaging prostate carcinoma.2010 Jan 6 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 6 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641373 Free Books & Documents. Review.
-
Chalcogen Atom-Modulated Croconaine for Efficient NIR-II Photothermal Theranostics.ACS Appl Mater Interfaces. 2024 Mar 13;16(10):12332-12338. doi: 10.1021/acsami.4c02254. Epub 2024 Mar 1. ACS Appl Mater Interfaces. 2024. PMID: 38426453
-
VivoTag-S680–conjugated 3-aminomethyl αvβ3 antagonist derivative for fluorescence molecular tomography of tumors.2010 Jan 6 [updated 2010 May 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 6 [updated 2010 May 19]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641993 Free Books & Documents. Review.
Cited by
-
Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable.Pharmaceutics. 2022 Aug 15;14(8):1697. doi: 10.3390/pharmaceutics14081697. Pharmaceutics. 2022. PMID: 36015322 Free PMC article. Review.
-
Nanotechnology for angiogenesis: opportunities and challenges.Chem Soc Rev. 2020 Jul 21;49(14):5008-5057. doi: 10.1039/c8cs01021h. Epub 2020 Jun 15. Chem Soc Rev. 2020. PMID: 32538379 Free PMC article. Review.
-
Photothermal Therapy for the Treatment of Glioblastoma: Potential and Preclinical Challenges.Front Oncol. 2021 Jan 15;10:610356. doi: 10.3389/fonc.2020.610356. eCollection 2020. Front Oncol. 2021. PMID: 33520720 Free PMC article. Review.
-
Dynamic diselenide bond-enabled liquid crystal elastomer-based two-way shape memory aerogels with weldability and closed-loop recyclability.Smart Mol. 2023 Oct 12;1(3):e20230009. doi: 10.1002/smo.20230009. eCollection 2023 Dec. Smart Mol. 2023. PMID: 40626211 Free PMC article.
-
Soft nano and microstructures for the photomodulation of cellular signaling and behavior.Adv Drug Deliv Rev. 2022 Nov;190:114554. doi: 10.1016/j.addr.2022.114554. Epub 2022 Sep 28. Adv Drug Deliv Rev. 2022. PMID: 36181993 Free PMC article. Review.
References
-
- Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional Nanoparticles for Nultimodal Imaging and Theragnosis. Chem Soc Rev. 2012;41(7):2656–2672. - PubMed
-
- Zhao J, Yang Y, Han X, Liang C, Liu J, Song X, Ge Z, Liu Z. Redox-Sensitive Nanoscale Coordination Polymers for Drug Delivery and Cancer Theranostics. Acs Appl Mater Interfaces. 2017;9(28):23555–23563. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
