Bio-inspired melanin nanoparticles induce cancer cell death by iron adsorption
- PMID: 30506101
- PMCID: PMC6267116
- DOI: 10.1007/s10856-018-6190-x
Bio-inspired melanin nanoparticles induce cancer cell death by iron adsorption
Abstract
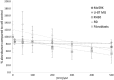
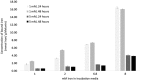
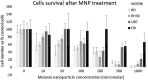
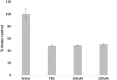
Dysregulation of iron metabolism is a common characteristic of cancer cells. The rapid proliferation of the tumour cells means that there is an increased dependence upon iron compared to healthy cells. Chelation of iron can be undertaken with a number of different compounds, however, simply lowering systemic iron levels to control tumour growth is not possible since iron is essential for cellular metabolism in the rest of the body. Nanoparticulate iron chelators could overcome this difficulty by targeting to the tumour either by the passive enhanced permeation and retention effect, or by targeting ligands on the surface. Nanoparticles were prepared from melanin, which is a naturally occurring pigment that is widely distributed within the body, but that can chelate iron. The prepared nanoparticles were shown to be ~220 nm, and could adsorb 16.45 mmoles iron/g melanin. The nanoparticles showed no affect on control fibroblast cells at a concentration of 200 μM, whereas the immortalised cancer cell lines showed at least 56% reduction in cell growth. At a concentration of 1 mM melanin nanoparticles the cell growth could be reduced by 99% compared to the control. The nanoparticles also show no significant haemotoxicity, even at concentration of 500 μM. Melanin nanoparticles are therefore a viable prospect for destroying cancer cells via iron starvation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

