A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study
- PMID: 30506110
- PMCID: PMC6418299
- DOI: 10.1007/s10549-018-5058-6
A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study
Abstract
Purpose: Cardiac dysfunction risk associated with intravenous trastuzumab (H IV) treatment may differ in real-world practice versus randomized trials. We investigated cardiac events in patients with HER2-positive early breast cancer (EBC) treated with H IV as adjuvant therapy in routine practice.
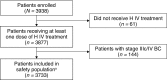
Methods: The observational study of cardiac events in patients with HER2-positive EBC treated with Herceptin (OHERA; NCT01152606) enrolled patients with stage I-IIIb disease eligible for H IV in the adjuvant setting per the European Summary of Product Characteristics (SmPC). Primary outcomes were symptomatic congestive heart failure incidence (CHF; New York Heart Association class II-IV) and cardiac death. Patient visits/assessments were per local practice.
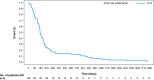
Results: 3733 Patients received ≥ 1 H IV dose per local practice; 88.9% received H IV for > 300 days (median follow-up: ~ 5 years). Prior to disease recurrence (if any), symptomatic CHF occurred in 106 patients (2.8%); 6 (0.2%) cardiac deaths occurred (5 in patients with cardiac disease history). Median time to symptomatic CHF onset was 5.7 months (95% CI 5.3-6.5); 77/106 (72.6%) patients with symptomatic CHF achieved resolution. CHF incidence was higher in patients ≥ 65 years, and those with pre-existing cardiac conditions, hypertension, or left ventricular ejection fraction ≤ 55% at baseline.
Conclusions: OHERA is the largest prospective observational study to investigate the cardiac safety of H IV as adjuvant EBC therapy in a real-world setting. Symptomatic CHF and cardiac event incidences were consistent with randomized trials in this setting and baseline risk factors identified in the H IV European SmPC.
Keywords: Cardiac adverse events; HER2-positive early breast cancer; HER2-targeted therapies; Real-world population; Trastuzumab.
Conflict of interest statement
Conflict of interest
The OHERA study was sponsored by F. Hoffmann-La Roche Ltd., Basel, Switzerland. Dr. Lidbrink reports Consultancy/Advisory role for F. Hoffmann-La Roche. Dr. Chmielowska reports research funding from F. Hoffmann-La Roche. Dr. Bouhlel, Mr. Liste Hermoso, and Dr. Nüesch are employees of F. Hoffmann-La Roche Ltd. Dr. Bouhlel and Dr. Nüesch report stock in F. Hoffmann-La Roche Ltd. Dr. Lauer reports consultancy for F. Hoffmann-La Roche Ltd. Dr. Shing was an employee of Genentech, Inc., at the time of this study and reports remuneration and consultancy for Genentech, Inc. Dr. Misra reports Consultancy/Advisory role for Eisai, Amgen, and Pfizer. Dr. Otremba reports no financial conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures

References
-
- Roche Registration Ltd. Herceptin® (trastuzumab). Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed Sep 2018
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

