Short-term changes observed in multiparametric liver MRI following therapy with direct-acting antivirals in chronic hepatitis C virus patients
- PMID: 30506214
- PMCID: PMC6510871
- DOI: 10.1007/s00330-018-5788-1
Short-term changes observed in multiparametric liver MRI following therapy with direct-acting antivirals in chronic hepatitis C virus patients
Abstract
Methods: We applied multiparametric MRI to assess changes in liver composition, perfusion and blood flow in 17 patients before direct-acting antiviral (DAA) therapy and after treatment completion (within 12 weeks of last DAA tablet swallowed).
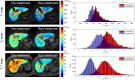
Results: We observed changes in hepatic composition indicated by a reduction in both liver longitudinal relaxation time (T1, 35 ± 4 ms), transverse relaxation time (T2, 2.5 ± 0.8 ms; T2* 3.0 ± 0.7 ms), and liver perfusion (28.1 ± 19.7 ml/100 g/min) which we suggest are linked to reduced pro-inflammatory milieu, including interstitial oedema, within the liver. No changes were observed in liver or spleen blood flow, splenic perfusion, or superior mesenteric artery blood flow.
Conclusion: For the first time, our study has shown that treatment of HCV with DAAs in patients with cirrhosis leads to an acute reduction in liver T1, T2 and T2* and an increase in liver perfusion measured using MR parameters. The ability of MRI to characterise changes in the angio-architecture of patients with cirrhosis after intervention in the short term will enhance our understanding of the natural history of regression of liver disease and potentially influence clinical decision algorithms.
Key points: • DAAs have revolutionised the treatment of hepatitis C and achieve sustained virological response in over 95% of patients, even with liver cirrhosis. • Currently available non-invasive measures of liver fibrosis are not accurate after HCV treatment with DAAs, this prospective single-centre study has shown that MRI can sensitively measure changes within the liver, which could reflect the reduction in inflammation with viral clearance. • The ability of MRI to characterise changes in structural and haemodynamic MRI measures in the liver after intervention will enhance our understanding of the progression/regression of liver disease and could potentially influence clinical decision algorithms.
Keywords: Echo-planar imaging; Hepatitis C; Magnetic resonance imaging; Sustained virologic response.
Conflict of interest statement
Guarantor
The scientific guarantor of this publication is Professor Susan Francis.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
The HCV MRI study received ethical approval by the NRES Committee East Midlands - Derby 1 (Research Ethics Committee reference 11/EM/0314).
Methodology
• Prospective
• Observational
• Performed at one institution
Figures



References
-
- Polaris Observatory HCV Collaborators (2017) Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2:161–176 - PubMed
-
- Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. - DOI - PubMed
-
- Foster GR, Irving WL, Cheung MC et al (2016) Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 64:1224–1231 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

