A Videogame-Based Digital Therapeutic to Improve Processing Speed in People with Multiple Sclerosis: A Feasibility Study
- PMID: 30506301
- PMCID: PMC6534643
- DOI: 10.1007/s40120-018-0121-0
A Videogame-Based Digital Therapeutic to Improve Processing Speed in People with Multiple Sclerosis: A Feasibility Study
Abstract
Introduction: Self-administered in-home digital therapeutics could expand access to cognitive rehabilitation for individuals with multiple sclerosis (MS), over half of whom experience cognitive impairment (CI). However, feasibility in an MS population must be clarified. This study was conducted to assess the feasibility of deploying a videogame-like digital treatment for CI in MS, including initial efficacy and barriers to adherence.
Methods: In this pilot study, 21 participants with MS completed an in-clinic baseline neurological evaluation. Cognitive tests included paper-and-pencil Brief International Cognitive Assessment for Multiple Sclerosis [BICAMS-which included the Symbol Digit Modalities Test (SDMT)] and other unsupervised tablet-based tests (including Match: an unsupervised test of executive functions and processing speed, developed at UCSF; and the Cogstate MS Battery). Participants then completed an in-home, tablet-based, videogame-like investigational digital treatment (Project: EVO™) for 25 min daily, 5 days weekly, for 4 weeks. This was followed by a repeat in-clinic evaluation.
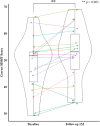
Results: Of the 21 participants (mean [standard deviation, SD] age 53.8 [11.6] years, median Expanded Disability Status Scale (EDSS) 2.5 [SD 2.0, IQR [2-3.5]]) enrolled to use the digital therapeutic at home (mean [SD] SDMT z score: - 0.21 [1.16]), 18 completed the study, during which they completed an average of 19.7 days (median [SD]: 20.5 [8.4]). Overall, 78% of these 18 participants completed 75% of prescribed days (i.e., at least 15), and 50% completed all 20 days or more. Over the 4-week period, scores of processing speed improved significantly (based on one-sided t test), including SDMT (p = 0.003) and Match (p = 0.006). The Cogstate DET test (psychomotor function) also increased (p = 0.006). Mean increase in SDMT was 3.6 points. Male sex, not being employed, and higher baseline anxiety all were significantly associated with greater improvement in SDMT over the 4-week period. Interestingly, lower baseline cognitive scores were associated with greater number of sessions completed (e.g., SDMT: p = 0.003, R2 = 0.44). Adjusting for employment, a proxy for time available, did not significantly improve the model fit.
Discussion: Deploying an in-home digital tool to improve processing speed in MS is feasible, and shows preliminary efficacy. A larger, randomized controlled clinical trial is ongoing.
Keywords: Cognition; Digital health; MHEALTH; Multiple Sclerosis; Processing speed.