Effect of DJ-1 on the neuroprotection of astrocytes subjected to cerebral ischemia/reperfusion injury
- PMID: 30506316
- PMCID: PMC6348070
- DOI: 10.1007/s00109-018-1719-5
Effect of DJ-1 on the neuroprotection of astrocytes subjected to cerebral ischemia/reperfusion injury
Abstract
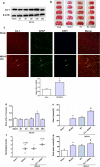
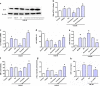
Astrocytes are involved in neuroprotection, and DJ-1 is an important antioxidant protein that is abundantly expressed in reactive astrocytes. However, the role of DJ-1 in astrocytes' neuroprotection in cerebral ischemia/reperfusion injury and its potential mechanism is unclear. Thus, to explore effects and mechanisms of DJ-1 on the neuroprotection of astrocytes, we used primary co-cultures of neurons and astrocytes under oxygen and glucose deprivation/reoxygenation in vitro and transient middle cerebral artery occlusion/reperfusion in vivo to mimic ischemic reperfusion insult. Lentiviral was used to inhibit and upregulate DJ-1 expression in astrocytes, and DJ-1 siRNA blocked DJ-1 expression in rats. Inhibiting DJ-1 expression led to decreases in neuronal viability. DJ-1 knockdown also attenuated total and nuclear Nrf2 and glutathione (GSH) levels in vitro and vivo. Similarly, loss of DJ-1 decreased Nrf2/ARE-binding activity and expression of Nrf2/ARE pathway-driven genes. Overexpression of DJ-1 yielded opposite results. This suggests that the mechanism of action of DJ-1 in astrocyte-mediated neuroprotection may involve regulation of the Nrf2/ARE pathway to increase GSH after cerebral ischemia/reperfusion injury. Thus, DJ-1 may be a new therapeutic target for treating ischemia/reperfusion injury. KEY MESSAGES: Astrocytes protect neurons in co-culture after OGD/R DJ-1 is upregulated in astrocytes and plays an important physiological roles in neuronal protection under ischemic conditions DJ-1 protects neuron by the Nrf2/ARE pathway which upregulates GSH.
Keywords: Astrocyte; Co-culture; DJ-1; GSH; I/R injury; Nrf2/ARE.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

