Prediction of therapy response in bone-predominant metastatic breast cancer: comparison of [18F] fluorodeoxyglucose and [18F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging
- PMID: 30506455
- PMCID: PMC6450846
- DOI: 10.1007/s00259-018-4223-9
Prediction of therapy response in bone-predominant metastatic breast cancer: comparison of [18F] fluorodeoxyglucose and [18F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging
Abstract
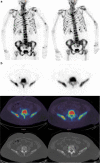
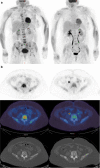
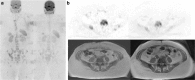
Purpose: To compare [18F]-fluorodeoxyglucose (FDG) and [18F]-sodium fluoride (NaF) positron emission tomography/computed tomography (PET/CT) with whole-body magnetic resonance with diffusion-weighted imaging (WB-MRI), for endocrine therapy response prediction at 8 weeks in bone-predominant metastatic breast cancer.
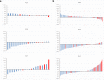
Patients and methods: Thirty-one patients scheduled for endocrine therapy had up to five bone metastases measured [FDG, NaF PET/CT: maximum standardized uptake value (SUVmax); WB-MRI: median apparent diffusion coefficient (ADCmed)] at baseline and 8 weeks. To detect the flare phenomenon, a 12-week NaF PET/CT was also performed if 8-week SUVmax increased. A 25% parameter change differentiated imaging progressive disease (PD) from non-PD and was compared to a 24-week clinical reference standard and progression-free survival (PFS).
Results: Twenty-two patients (median age, 58.6 years, range, 40-79 years) completing baseline and 8-week imaging were included in the final analysis. Per-patient % change in NaF SUVmax predicted 24-week clinical PD with sensitivity, specificity and accuracy of 60, 73.3, and 70%, respectively. For FDG SUVmax the results were 0, 100, and 76.2% and for ADCmed, 0, 100 and 72.2%, respectively. PFS < 24 weeks was associated with % change in SUVmax (NaF: 41.7 vs. 0.7%, p = 0.039; FDG: - 4.8 vs. - 28.6%, p = 0.005) but not ADCmed (- 0.5 vs. 10.1%, p = 0.098). Interlesional response heterogeneity occurred in all modalities and NaF flare occurred in seven patients.
Conclusions: FDG PET/CT and WB-MRI best predicted clinical non-PD and both FDG and NaF PET/CT predicted PFS < 24 weeks. Lesional response heterogeneity occurs with all modalities and flare is common with NaF PET/CT.
Keywords: Bone metastases; Diffusion-weighted MRI; Positron emission tomography/computed tomography; Whole-body MRI; [18F]-fluorodeoxyglucose; [18F]-sodium fluoride.
Conflict of interest statement
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Hoefeler H, Duran I, Hechmati G, Garzon Rodriguez C, Luftner D, Ashcroft J, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: results from a multinational retrospective—prospective observational study—a cohort from 4 European countries. J Bone Oncol. 2014;3:40–48. doi: 10.1016/j.jbo.2014.04.001. - DOI - PMC - PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the international Letrozole breast cancer group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

