Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial
- PMID: 30506499
- PMCID: PMC6342854
- DOI: 10.1007/s11060-018-03057-z
Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial
Abstract
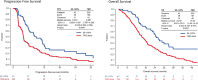
Background: Tumor treating fields (TTFields) is a non-invasive, antimitotic therapy. In the EF-14 phase 3 trial in newly diagnosed glioblastoma, TTFields plus temozolomide (TTFields/TMZ) improved progression free (PFS) and overall survival (OS) versus TMZ alone. Previous data indicate a ≥ 75% daily compliance improves outcomes. We analyzed compliance data from TTFields/TMZ patients in the EF-14 study to correlate TTFields compliance with PFS and OS and identify potential lower boundary for compliance with improved clinical outcomes.
Methods: Compliance was assessed by usage data from the NovoTTF-100A device and calculated as percentage per month of TTFields delivery. TTFields/TMZ patients were segregated into subgroups by percent monthly compliance. A Cox proportional hazard model controlled for sex, extent of resection, MGMT methylation status, age, region, and performance status was used to investigate the effect of compliance on PFS and OS.
Results: A threshold value of 50% compliance with TTFields/TMZ improved PFS (HR 0.70, 95% CI 0.47-1.05) and OS (HR 0.67, 95% CI 0.45-0.99) versus TMZ alone with improved outcome as compliance increased. At compliance > 90%, median survival was 24.9 months (28.7 months from diagnosis) and 5-year survival rate was 29.3%. Compliance was independent of gender, extent of resection, MGMT methylation status, age, region and performance status (HR 0.78; p = 0.031; OS at compliance ≥ 75% vs. < 75%).
Conclusion: A compliance threshold of 50% with TTFields/TMZ correlated with significantly improved OS and PFS versus TMZ alone. Patients with compliance > 90% showed extended median and 5-year survival rates. Increased compliance with TTFields therapy is independently prognostic for improved survival in glioblastoma.
Trial registration: ClinicalTrials.gov NCT00916409.
Keywords: Compliance; Glioblastoma; Monthly usage; Tumor treating fields.
Conflict of interest statement
S. A. Toms, C. Y. Kim and G. Nicholas have nothing to disclose. Z. Ram reports a research grant (principal investigator and consultant) with Novocure, Ltd. and ownership interest (stock) in Novocure, Ltd.
Figures


References
-
- Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-oncology. 2017;19:v1–v88. doi: 10.1093/neuonc/nox158. - DOI - PMC - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

