In vivo multiphoton microscopy of melasma
- PMID: 30506627
- PMCID: PMC6483848
- DOI: 10.1111/pcmr.12756
In vivo multiphoton microscopy of melasma
Abstract
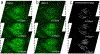
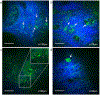
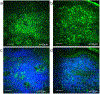
Melasma is a skin disorder characterized by hyperpigmented patches due to increased melanin production and deposition. In this pilot study, we evaluate the potential of multiphoton microscopy (MPM) to characterize non-invasively the melanin content, location, and distribution in melasma and assess the elastosis severity. We employed a clinical MPM tomograph to image in vivo morphological features in melasma lesions and adjacent normal skin in 12 patients. We imaged dermal melanophages in most dermal melasma lesions and occasionally in epidermal melasma. The melanin volume fraction values measured in epidermal melasma (14% ± 4%) were significantly higher (p < 0.05) than the values measured in perilesional skin (11% ± 3%). The basal keratinocytes of melasma and perilesions showed different melanin distribution. Elastosis was predominantly more severe in lesions than in perilesions and was associated with changes in melanin distribution of the basal keratinocytes. These results demonstrate that MPM may be a non-invasive imaging tool for characterizing melasma.
Keywords: in vivo imaging; melasma; microscopy.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures




References
-
- Ardigo M, Cameli N, Berardesca E, and Gonzalez S (2010). Characterization and evaluation of pigment distribution and response to therapy in melasma using in vivo reflectance confocal microscopy: a preliminary study. Journal of the European Academy of Dermatology and Venereology 24, 1296–1303. - PubMed

