Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits
- PMID: 30507615
- PMCID: PMC6307945
- DOI: 10.1172/JCI95731
Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits
Abstract
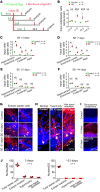
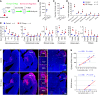
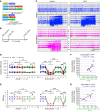
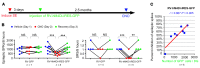
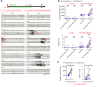
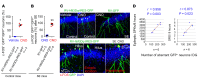
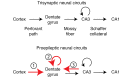
We investigated how pathological changes in newborn hippocampal dentate granule cells (DGCs) lead to epilepsy. Using a rabies virus-mediated retrograde tracing system and a designer receptors exclusively activated by designer drugs (DREADD) chemogenetic method, we demonstrated that newborn hippocampal DGCs are required for the formation of epileptic neural circuits and the induction of spontaneous recurrent seizures (SRS). A rabies virus-mediated mapping study revealed that aberrant circuit integration of hippocampal newborn DGCs formed excessive de novo excitatory connections as well as recurrent excitatory loops, allowing the hippocampus to produce, amplify, and propagate excessive recurrent excitatory signals. In epileptic mice, DREADD-mediated-specific suppression of hippocampal newborn DGCs dramatically reduced epileptic spikes and SRS in an inducible and reversible manner. Conversely, specific activation of hippocampal newborn DGCs increased both epileptic spikes and SRS. Our study reveals an essential role for hippocampal newborn DGCs in the formation and function of epileptic neural circuits, providing critical insights into DGCs as a potential therapeutic target for treating epilepsy.
Keywords: Epilepsy; Neuronal stem cells; Neuroscience; Stem cells.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

