MicroRNA characterization in equine induced pluripotent stem cells
- PMID: 30507934
- PMCID: PMC6277106
- DOI: 10.1371/journal.pone.0207074
MicroRNA characterization in equine induced pluripotent stem cells
Abstract
Cell reprogramming has been well described in mouse and human cells. The expression of specific microRNAs has demonstrated to be essential for pluripotent maintenance and cell differentiation, but not much information is available in domestic species. We aim to generate horse iPSCs, characterize them and evaluate the expression of different microRNAs (miR-302a,b,c,d, miR-205, miR-145, miR-9, miR-96, miR-125b and miR-296). Two equine iPSC lines (L2 and L3) were characterized after the reprogramming of equine fibroblasts with the four human Yamanaka's factors (OCT-4/SOX-2/c-MYC/KLF4). The pluripotency of both lines was assessed by phosphatase alkaline activity, expression of OCT-4, NANOG and REX1 by RT-PCR, and by immunofluorescence of OCT-4, SOX-2 and c-MYC. In vitro differentiation to embryo bodies (EBs) showed the capacity of the iPSCs to differentiate into ectodermal, endodermal and mesodermal phenotypes. MicroRNA analyses resulted in higher expression of the miR-302 family, miR-9 and miR-96 in L2 and L3 vs. fibroblasts (p<0.05), as previously shown in human pluripotent cells. Moreover, downregulation of miR-145 and miR-205 was observed. After differentiation to EBs, higher expression of miR-96 was observed in the EBs respect to the iPSCs, and also the expression of miR-205 was induced but only in the EB-L2. In addition, in silico alignments of the equine microRNAs with mRNA targets suggested the ability of miR-302 family to regulate cell cycle and epithelial mesenchymal transition genes, miR-9 and miR-96 to regulate neural determinant genes and miR-145 to regulate pluripotent genes, similarly as in humans. In conclusion, we could obtain equine iPSCs, characterize them and determine for the first time the expression level of microRNAs in equine pluripotent cells.
Conflict of interest statement
We have the following interests: Author Gabriel Vichera is employed by Kheiron Biotech. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
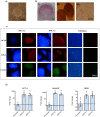


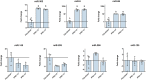
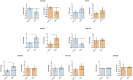

References
-
- Blomberg LA, Telugu BPVL. Twenty years of embryonic stem cell research in farm animals. Reprod Domest Anim. 2012;47 Suppl 4:80–5. 10.1111/j.1439-0531.2012.02059.x - DOI - PubMed
-
- Smith RKW, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 2013;8(9):e75697 10.1371/journal.pone.0075697 - DOI - PMC - PubMed
-
- Broeckx S, Zimmerman M, Crocetti S, Suls M, Mariën T, Ferguson SJ, et al. Regenerative therapies for equine degenerative joint disease: a preliminary study. PLoS One. 2014;9(1):e85917 10.1371/journal.pone.0085917 - DOI - PMC - PubMed
-
- Geburek F, Roggel F, van Schie HTM, Beineke A, Estrada R, Weber K, et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. 2017;8(1):129 10.1186/s13287-017-0564-8 - DOI - PMC - PubMed
-
- Egusa H, Kayashima H, Miura J, Uraguchi S, Wang F, Okawa H, et al. Comparative analysis of mouse-induced pluripotent stem cells and mesenchymal stem cells during osteogenic differentiation in vitro. Stem Cells Dev. 2014;23(18):2156–69. 10.1089/scd.2013.0344 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

