Correlation of In Vivo [18F]Flortaucipir With Postmortem Alzheimer Disease Tau Pathology
- PMID: 30508025
- PMCID: PMC6439731
- DOI: 10.1001/jamaneurol.2018.3692
Correlation of In Vivo [18F]Flortaucipir With Postmortem Alzheimer Disease Tau Pathology
Abstract
Importance: In Alzheimer disease (AD), tau filaments form neuronal inclusions in neurites (neuropil threads) and in somata (neurofibrillary tangles), and neurite tau pathology constitutes the most common pathology. Positron emission tomography (PET) ligands have been developed to detect in vivo tau pathology in AD. However, the association of AD tau pathology post mortem with in vivo tau PET retention has not been established. Therefore, there is a need to investigate the associations of tau PET with postmortem tau pathology in AD.
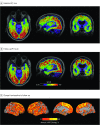
Objective: To study the association of regional in vivo retention of the tau PET ligand [18F]flortaucipir (previously known as AV1451) with the density of tau neuropathology in the corresponding brain regions in a patient with AD.
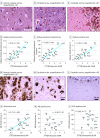
Design, setting, and participants: The patient was a man in his 40s with AD caused by a PSEN1 mutation. Between May 2015 and December 2016, he underwent 2 [18F]flortaucipir PET scans at Lund University Hospital, Lund, Sweden. Postmortem analysis was performed 12 months after the last PET scan. Tau pathology was assessed using phosphorylated tau (AT8) immunohistochemistry and Gallyas silver staining. In addition to the regional total tau pathology burden, the density of tau-positive neurites and intrasomal tau tangles were quantified using a stereology-based method. Further, β-amyloid-containing plaques were detected using 4G8 immunohistochemistry. Data were analyzed between January 2018 and August 2018.
Main outcomes and measures: Regional standardized uptake value ratios of [18F]flortaucipir were compared with the amount of tau pathology in the corresponding brain areas.
Results: In this patient, the clinical disease symptoms progressed rapidly in life, paralleled with an annual increase of tau PET retention of 20% to 40% in many cortical regions. Compared with postmortem immunohistochemistry, regional in vivo uptake of [18F]flortaucipir was correlated with the density of tau-positive neurites (AT8: rs = 0.87; P < .001; Gallyas: rs = 0.92; P < .001), intrasomal tau tangles (AT8: rs = 0.65; P = .01; Gallyas: rs = 0.84; P < .001), and total tau burden (AT8: rs = 0.84; P < .001; Gallyas: rs = 0.82; P < .001). No correlations between [18F]flortaucipir and β-amyloid pathology were found.
Conclusions and relevance: These results indicate that [18F]flortaucipir PET retention is a robust in vivo measure of the total AD tau burden.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

