Association of Seizure Spread With Surgical Failure in Epilepsy
- PMID: 30508033
- PMCID: PMC6459131
- DOI: 10.1001/jamaneurol.2018.4316
Association of Seizure Spread With Surgical Failure in Epilepsy
Abstract
Importance: Seizures recur in as many as half of patients who undergo surgery for drug-resistant temporal lobe epilepsy (TLE). Understanding why TLE is resistant to surgery in some patients may reveal insights into epileptogenic networks and direct new therapies to improve outcomes.
Objective: To characterize features of surgically refractory TLE.
Design, setting, and participants: Medical records from a comprehensive epilepsy center were retrospectively reviewed for 131 patients who received a standard anteromedial temporal resection by a single surgeon from January 1, 2000, to December 31, 2015. Thirteen patients were excluded for having less than 1 year of follow-up. Patients at the highest risk for seizure recurrence were identified. Intracranial electroencephalogram (iEEG) analyses generated 3-dimensional seizure spread representations and quantified rapid seizure spread. The final analyses of seizure outcome and follow-up data were performed in June 2017.
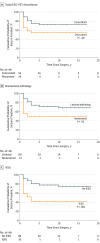
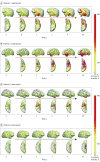
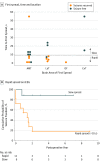
Main outcomes and measures: The Engel class seizure outcome following surgery was evaluated for all patients, defining seizure recurrence as Engel class II or greater. Intracranial recordings of neocortical grids/strips and depth electrodes were analyzed visually for seizure spread. Fast β power was projected onto reconstructions of patients' brain magnetic resonance imaging scans to visualize spread patterns and was quantified to compare power within vs outside resective margins.
Results: Of 118 patients with 1 year of follow-up or more (mean [SD], 6.5 [4.6] years), 66 (55.9%) were women and 52 (44.1%) were men (median age, 39 years [range, 4-66 years]). The cumulative probability of continuous Engel class I seizure freedom since surgery at postoperative year 10 and afterward was 65.6%, with 92% of recurrences in years 1 to 3. Multivariable statistical analyses found that the selection for iEEG study was the most reliable predictor of seizure recurrence, with a mixed-effects model estimating that the Engel score in the iEEG cohort was higher by a mean (SD) of 1.1 (0.33) (P = .001). In patients with iEEG results, rapid seizure spread in less than 10 seconds was associated with recurrence (hazard ratio, 5.99; 95% CI, 1.7-21.1; P < .01). In the first 10 seconds of seizures, fast β power activity outside the resective margins in the lateral temporal cortex was significantly greater in patients whose seizures recurred compared with patients who were seizure-free (mean [SEM], 137.5% [16.8%] vs 93.4% [4.6%]; P < .05).
Conclusions and significance: Rapid seizure spread outside anteromedial temporal resection resective margins plays a significant role in the surgical failure of drug-resistant TLE. Seizure control after epilepsy surgery might be improved by investigating areas of early spread as candidates for resection or neuromodulation.
Conflict of interest statement
Figures