Detection of Apoptotic Circulating Tumor Cells Using in vivo Fluorescence Flow Cytometry
- PMID: 30508273
- PMCID: PMC9257981
- DOI: 10.1002/cyto.a.23642
Detection of Apoptotic Circulating Tumor Cells Using in vivo Fluorescence Flow Cytometry
Abstract
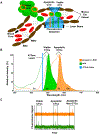
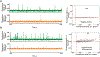
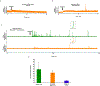
Most cancer patients die from metastatic disease as a result of a circulating tumor cell (CTC) spreading from a primary tumor through the blood circulation to distant organs. Many studies have demonstrated the tremendous potential of using CTC counts as prognostic markers of metastatic development and therapeutic efficacy. However, it is only the viable CTCs capable of surviving in the blood circulation that can create distant metastasis. To date, little progress has been made in understanding what proportion of CTCs is viable and what proportion is in an apoptotic state. Here, we introduce a novel approach toward in situ characterization of CTC apoptosis status using a multicolor in vivo flow cytometry platform with fluorescent detection for the real-time identification and enumeration of such cells directly in blood flow. The proof of concept was demonstrated with two-color fluorescence flow cytometry (FFC) using breast cancer cells MDA-MB-231 expressing green fluorescein protein (GFP), staurosporine as an activator of apoptosis, Annexin-V apoptotic kit with orange dye color, and a mouse model. The future application of this new platform for real-time monitoring of antitumor drug efficiency is discussed. © 2018 International Society for Advancement of Cytometry.
Keywords: apoptosis; circulating tumor cells; fluorescence flow cytometry; in vivo detection of circulating tumor cells.
© 2018 International Society for Advancement of Cytometry.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

