Clinical relevance of guanine-derived urinary biomarkers of oxidative stress, determined by LC-MS/MS
- PMID: 30508700
- PMCID: PMC6279954
- DOI: 10.1016/j.redox.2018.11.016
Clinical relevance of guanine-derived urinary biomarkers of oxidative stress, determined by LC-MS/MS
Abstract
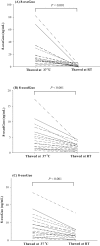
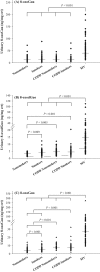
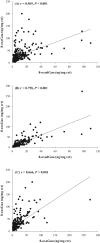
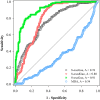
A reliable and fast liquid chromatography-tandem mass spectrometry method has been developed for the simultaneous determination of three oxidized nucleic acid damage products in urine, 8-oxoguanine (8-oxoGua), 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodGuo) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo). We applied this method to assess the effect of various urine workup procedures on the urinary concentrations of the oxidized nucleic acid products. Our results showed that frozen urine samples must be warmed (i.e., to 37 °C) to re-dissolve any precipitates prior to analysis. We showed that common workup procedures, such as thawing at room temperature or dilution with deionized water, are not capable of releasing fully the oxidized nucleic acid products from the precipitates, and result in significant underestimation (up to ~ 100% for 8-oxoGua, ~ 86% for both 8-oxodGuo and 8-oxoGuo). With this method, we further assessed and compared the ability of the three oxidized nucleic acid products, as well as malondialdehyde (MDA, a product of lipid peroxidation), to biomonitor oxidative stress in vivo. We measured a total of 315 urine samples from subjects with burdens of oxidative stress from low to high, including healthy subjects, patients with chronic obstructive pulmonary disease (COPD), and patients on mechanical ventilation (MV). The results showed that both the MV and COPD patients had significantly higher urinary levels of 8-oxoGua, 8-oxodGuo, and 8-oxoGuo (P < 0.001), but lower MDA levels, compared to healthy controls. Receiver operating characteristic curve analysis revealed that urinary 8-oxoGuo is the most sensitive biomarker for oxidative stress with area under the curve (AUC) of 0.91, followed by 8-oxodGuo (AUC: 0.80) and 8-oxoGua (AUC: 0.76). Interestingly, MDA with AUC of 0.34 failed to discriminate the patients from healthy controls. Emerging evidence suggests a potential clinical utility for the measurement of urinary 8-oxoGuo, and to a lesser extent 8-oxodGuo, which is strongly supported by our findings.
Keywords: COPD; LC-MS/MS; Lipid peroxidation; Mechanical ventilation; Nucleic acid oxidation; Urine precipitates.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Olinski R., Gackowski D., Cooke M.S. Endogenously generated DNA nucleobase modifications source, and significance as possible biomarkers of malignant transformation risk, and role in anticancer therapy. Biochim. Biophys. Acta. 1869;2018:29–41. - PubMed
-
- Neeley W.L., Essigmann J.M. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 2006;19:491–505. - PubMed
-
- Cheng K.C., Cahill D.S., Kasai H., Nishimura S., Loeb L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J. Biol. Chem. 1992;267:166–172. - PubMed
-
- Brancato B., Munnia A., Cellai F., Ceni E., Mello T., Bianchi S., Catarzi S., Risso G.G., Galli A., Peluso M.E. 8-Oxo-7,8-dihydro-2′-deoxyguanosine and other lesions along the coding strand of the exon 5 of the tumour suppressor gene P53 in a breast cancer case-control study. DNA Res. 2016;23:395–402. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

