Performance of a DNA methylation marker panel using liquid-based cervical scrapes to detect cervical cancer and its precancerous stages
- PMID: 30509219
- PMCID: PMC6276155
- DOI: 10.1186/s12885-018-5125-8
Performance of a DNA methylation marker panel using liquid-based cervical scrapes to detect cervical cancer and its precancerous stages
Abstract
Background: A change of cervical cancer screening algorithms to an HPV-based screening setting is discussed in many countries, due to higher sensitivity of HPV testing compared to cytology. Reliable triage methods are, however, an essential prerequisite in such a setting to avoid overtreatment and higher screening costs.
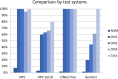
Results: In this study, a series of cervical scrapes collected in PreservCyt liquid-based cytology (LBC) medium from women with cervical cancer (n = 5), cervical intraepithelial neoplasia grade 1-3 (n = 74), and normal cytology (n = 201; further n = 352 collected in SureThin®) were assessed for methylation of the marker regions ASTN1, DLX1, ITGA4, RXFP3, SOX17, and ZNF671 using the GynTect assay and compared to cobas® HPV and CINtec Plus® biomarker results. All samples from women with cervical cancer, 61.2% of CIN3, 44.4% of CIN2 and 20.0% of CIN1 cases were scored positive for the GynTect methylation assay. In contrast, all CIN, irrespective of severity grade, and carcinomas were positive by both, CINtec Plus and cobas HPV. The specificity of GynTect for CIN3+ was 94.6% compared to 69.9% for CINtec Plus and 82.6% for cobas HPV (all HPV types) and 90.6% for cobas HPV 16/18. DNA methylation analysis of this methylation marker panel (GynTect assay) in cervical scrapes consistently detects cervical cancer and the majority of CIN3 as well as a subset of CIN1/2 lesions. The detection rate among cytologically normal samples is extraordinarily low (1.5%).
Conclusion: GynTect shows excellent performance when using cervical scrape material collected in liquid-based cytology media, a prerequisite for employing such a test as a triage in screening programs. Compared to the other test systems used in this work, GynTect showed higher specificity while still detecting all cancer cases.
Keywords: Biomarkers; Cervical cancer; DNA methylation; Epigenetic markers; Human papillomavirus (HPV).
Conflict of interest statement
Ethics approval and consent to participate
This study was designed as a quality development study, utilizing only residual material that otherwise would have been discarded. All patient samples were surplus samples and completely anonymized prior to use in the study. The study was performed following the guideline of the Declaration of Helsinki. According to German law an ethical approval is not required if using completely anonymized surplus samples in such a retrospective study setting.
Consent for publication
Not applicable.
Competing interests
MS, AH, KE and DS are shareholders and/or employees of oncgnostics GmbH, a company that aims to commercialize DNA methylation markers.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Anttila A, Ronco G, Working group on the R, monitoring of cervical Cancer screening Programmes in the European U, within the European network for information on C Description of the national situation of cervical cancer screening in the member states of the European Union. Eur J Cancer. 2009;45(15):2685–2708. doi: 10.1016/j.ejca.2009.07.017. - DOI - PubMed
-
- Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. - DOI - PubMed
-
- Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, Segnan N, Tomatis M, Soerjomataram I, Primic Zakelj M, et al. Status of implementation and organization of cancer screening in the European Union member states-summary results from the second European screening report. Int J Cancer. 2018;142(1):44–56. doi: 10.1002/ijc.31043. - DOI - PubMed
-
- Jaisamrarn U, Castellsague X, Garland SM, Naud P, Palmroth J, Del Rosario-Raymundo MR, Wheeler CM, Salmeron J, Chow SN, Apter D, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS One. 2013;8(11):e79260. doi: 10.1371/journal.pone.0079260. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical