C-Reactive Protein (CRP) levels in neonatal meningitis in England: an analysis of national variations in CRP cut-offs for lumbar puncture
- PMID: 30509228
- PMCID: PMC6276241
- DOI: 10.1186/s12887-018-1354-x
C-Reactive Protein (CRP) levels in neonatal meningitis in England: an analysis of national variations in CRP cut-offs for lumbar puncture
Abstract
Background: Recent National Institute for Health and Care Excellence (NICE) CG149 guidelines suggest considering performing a lumbar puncture (LP) to investigate for meningitis in early-onset sepsis in a neonate when a C-reactive protein (CRP) level >10mg/L, but the evidence for this recommendation is poorly defined.
Methods: Data on trust-wide LP protocols, neonatal meningitis incidence, lumbar punctures, and CRP levels seen in cases of neonatal meningitis were asked of all 137 trusts in England that recorded a birth in 2017. Our local Kingston Hospital data on every LP performed was obtained to estimate the specificity of CRP rises.
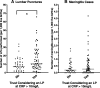
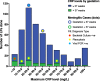
Results: 73/123 (59.3%) of trusts follow the NICE CG149 recommendation of considering an LP if the CRP >10mg/L. The national incidence of neonatal meningitis was 0.467/1,000 births, and an LP was performed in 1.37% of all babies, which was significantly higher in trusts considering the CRP > 10mg/L cut-off. A CRP > 10mg/L cut-off sensitivity was 88.9% based on the highest CRP level 4 days around the LP from national data of 199 cases; specificity was 78.8% based on our single-unit analysis.
Conclusions: Proposing a universal CRP > 10mg/L cut-off for a lumbar puncture has been counter-productive in England. Following it generates significantly more LPs, to the point that 40.7% of trusts have chosen not to follow it. It also has poor sensitivity missing over 11% of meningitis. We therefore do not recommend a universal cut-off, rather considering the whole clinical picture (including prematurity) when considering whether to do an LP.
Keywords: C-reactive protein; CG149; CRP; cerebrospinal fluid; cut-off; lumbar puncture; meningitis; neonatal; neonatal meningitis.
Conflict of interest statement
Ethics approval and consent to participate
As the national data involved is anonymised publicly-available data, no ethical opinion from an ethics committee was sought, which is in line with current views on ethical requirements of freedom of information act data[30]. The local Kingston data was collected by the clinical care team and then anonymised for the purposes of this project. The local Research & Development office has confirmed that no ethical approval or consent was required for this project; the local data released has been approved by the trust’s Caldecott guardian.
Consent for publication
No individually identifiable data is used, so no specific consent for publication has been sought.
Competing interests
JS is an Academic Clinical Fellow in paediatrics, which is funded by the NIHR.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Heath PT, Okike IO, Oeser C. Neonatal Meningitis: Can We Do Better? In: Curtis N, Finn A, Pollard AJ, editors. Hot Topics in Infection and Immunity in Children VIII. New York: Springer New York; 2011. pp. 11–24.
-
- Polin RA. Management of Neonates With Suspected or Proven Early-Onset Bacterial Sepsis: Pediatrics; 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

