Exploration of the Sialic Acid World
- PMID: 30509400
- PMCID: PMC7112061
- DOI: 10.1016/bs.accb.2018.09.001
Exploration of the Sialic Acid World
Abstract
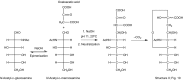
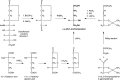
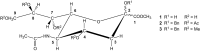
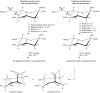
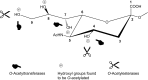
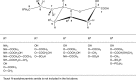
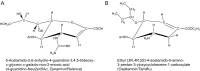
Sialic acids are cytoprotectors, mainly localized on the surface of cell membranes with multiple and outstanding cell biological functions. The history of their structural analysis, occurrence, and functions is fascinating and described in this review. Reports from different researchers on apparently similar substances from a variety of biological materials led to the identification of a 9-carbon monosaccharide, which in 1957 was designated "sialic acid." The most frequently occurring member of the sialic acid family is N-acetylneuraminic acid, followed by N-glycolylneuraminic acid and O-acetylated derivatives, and up to now over about 80 neuraminic acid derivatives have been described. They appeared first in the animal kingdom, ranging from echinoderms up to higher animals, in many microorganisms, and are also expressed in insects, but are absent in higher plants. Sialic acids are masks and ligands and play as such dual roles in biology. Their involvement in immunology and tumor biology, as well as in hereditary diseases, cannot be underestimated. N-Glycolylneuraminic acid is very special, as this sugar cannot be expressed by humans, but is a xenoantigen with pathogenetic potential. Sialidases (neuraminidases), which liberate sialic acids from cellular compounds, had been known from very early on from studies with influenza viruses. Sialyltransferases, which are responsible for the sialylation of glycans and elongation of polysialic acids, are studied because of their significance in development and, for instance, in cancer. As more information about the functions in health and disease is acquired, the use of sialic acids in the treatment of diseases is also envisaged.
Keywords: History; Sialic acids; Sialobiochemistry; Sialobiology; Sialochemistry.
© 2018 Elsevier Inc. All rights reserved.
Figures





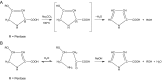
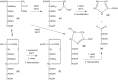
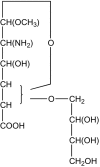

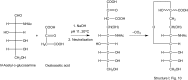



















References
-
- Montreuil J., Vliegenthart J.F.G., Schachter H. In: Glycoproteins II. Montreuil J., Vliegenthart J.F.G., Schachter H., editors. Vol. 29b. Elsevier Science B.V; Amsterdam, The Netherlands: 1997. Preface; pp. v–vi. (New Comprehensive Biochemistry).
-
- Warren L. The Distribution of Sialic Acids in Nature. Comp. Biochem. Physiol. 1963;10:153–171. - PubMed
-
- Warren L. In: Biological Roles of Sialic Acid. Rosenberg A., Schengrund C.-L., editors. Plenum Press; New York, NY, USA: 1976. The Distribution of Sialic Acids Within the Eukaryotic Cell; pp. 103–121.
-
- Ng S.-S., Dain J.A. In: Biological Roles of Sialic Acid. Rosenberg A., Schengrund C.-L., editors. Plenum Press; New York, NY, USA: 1976. The Natural Occurrence of Sialic Acids; pp. 59–102.
-
- Corfield A.P., Schauer R. In: Sialic Acids—Chemistry, Metabolism and Function. Schauer R., editor. Vol. 10. Springer-Verlag; Vienna, Austria: 1982. Occurrence of Sialic Acids; pp. 5–50. (Cell Biology Monographs).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

