Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial
- PMID: 30509743
- PMCID: PMC6294828
- DOI: 10.1016/S0140-6736(18)31813-0
Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial
Abstract
Background: Infant pain has immediate and long-term effects but is undertreated because of a paucity of evidence-based analgesics. Although morphine is often used to sedate ventilated infants, its analgesic efficacy is unclear. We aimed to establish whether oral morphine could provide effective and safe analgesia in non-ventilated premature infants for acute procedural pain.
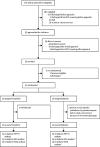
Methods: In this single-centre masked trial, 31 infants at the John Radcliffe Hospital, Oxford, UK, were randomly allocated using a web-based facility with a minimisation algorithm to either 100 μg/kg oral morphine sulphate or placebo 1 h before a clinically required heel lance and retinopathy of prematurity screening examination, on the same occasion. Eligible infants were born prematurely at less than 32 weeks' gestation or with a birthweight lower than 1501 g and had a gestational age of 34-42 weeks at the time of the study. The co-primary outcome measures were the Premature Infant Pain Profile-Revised (PIPP-R) score after retinopathy of prematurity screening and the magnitude of noxious-evoked brain activity after heel lancing. Secondary outcome measures assessed physiological stability and safety. This trial is registered with the European Clinical Trials Database (number 2014-003237-25).
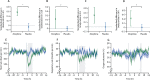
Findings: Between Oct 30, 2016, and Nov 17, 2017, 15 infants were randomly allocated to morphine and 16 to placebo; one infant assigned placebo was withdrawn from the study before monitoring began. The predefined stopping boundary was crossed, and trial recruitment stopped because of profound respiratory adverse effects of morphine without suggestion of analgesic efficacy. None of the co-primary outcome measures differed significantly between groups. PIPP-R score after retinopathy of prematurity screening was mean 11·1 (SD 3·2) with morphine and 10·5 (3·4) with placebo (mean difference 0·5, 95% CI -2·0 to 3·0; p=0·66). Noxious-evoked brain activity after heel lancing was median 0·99 (IQR 0·40-1·56) with morphine and 0·75 (0·33-1·22) with placebo (median difference 0·25, 95% CI -0·16 to 0·80; p=0·25).
Interpretation: Administration of oral morphine (100 μg/kg) to non-ventilated premature infants has the potential for harm without analgesic efficacy. We do not recommend oral morphine for retinopathy of prematurity screening and strongly advise caution if considering its use for other acute painful procedures in non-ventilated premature infants.
Funding: Wellcome Trust and National Institute for Health Research.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Use of morphine before retinopathy of prematurity examinations.Lancet. 2018 Dec 15;392(10164):2521-2523. doi: 10.1016/S0140-6736(18)32324-9. Epub 2018 Nov 30. Lancet. 2018. PMID: 30509742 No abstract available.
-
A universal right to pain relief: balancing the risks in a vulnerable patient population.Lancet Child Adolesc Health. 2019 Feb;3(2):62-64. doi: 10.1016/S2352-4642(18)30269-4. Epub 2018 Dec 1. Lancet Child Adolesc Health. 2019. PMID: 30509894 No abstract available.
-
Morphine compared to placebo for procedural pain in preterm infants: safety, efficacy and equipoise.J Perinatol. 2019 Oct;39(10):1428-1431. doi: 10.1038/s41372-019-0476-9. Epub 2019 Aug 27. J Perinatol. 2019. PMID: 31455824 Free PMC article. No abstract available.
References
-
- Roofthooft DWE, Simons SHP, Anand KJS, Tibboel D, van Dijk M. Eight years later, are we still hurting newborn infants? Neonatology. 2014;105:218–226. - PubMed
-
- Baarslag MA, Allegaert K, Van Den Anker JN. Paracetamol and morphine for infant and neonatal pain; still a long way to go? Expert Rev Clin Pharmacol. 2017;10:111–126. - PubMed
-
- Bellu R, de Waal K, Zanini R. Opioids for neonates receiving mechanical ventilation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2010;95:F241–F251. - PubMed
-
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. - PubMed
-
- Rahi JS, Cable N. Severe visual impairment and blindness in children in the UK. Lancet. 2003;362:1359–1365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

