Antibiotics Stimulate Formation of Vesicles in Staphylococcus aureus in both Phage-Dependent and -Independent Fashions and via Different Routes
- PMID: 30509943
- PMCID: PMC6355553
- DOI: 10.1128/AAC.01439-18
Antibiotics Stimulate Formation of Vesicles in Staphylococcus aureus in both Phage-Dependent and -Independent Fashions and via Different Routes
Abstract
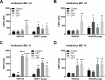
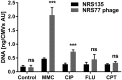
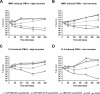
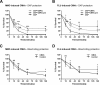
Bacterial membrane vesicle research has so far focused mainly on Gram-negative bacteria. Only recently have Gram-positive bacteria been demonstrated to produce and release extracellular membrane vesicles (MVs) that contribute to bacterial virulence. Although treatment of bacteria with antibiotics is a well-established trigger of bacterial MV formation, the underlying mechanisms are poorly understood. In this study, we show that antibiotics can induce MVs through different routes in the important human pathogen Staphylococcus aureus DNA-damaging agents and antibiotics inducing the SOS response triggered vesicle formation in lysogenic strains of S. aureus but not in their phage-devoid counterparts. The β-lactam antibiotics flucloxacillin and ceftaroline increased vesicle formation in a prophage-independent manner by weakening the peptidoglycan layer. We present evidence that the amount of DNA associated with MVs formed by phage lysis is greater than that for MVs formed by β-lactam antibiotic-induced blebbing. The purified MVs derived from S. aureus protected the bacteria from challenge with daptomycin, a membrane-targeting antibiotic, both in vitro and ex vivo in whole blood. In addition, the MVs protected S. aureus from killing in whole blood, indicating that antibiotic-induced MVs function as a decoy and thereby contribute to the survival of the bacterium.
Keywords: Staphylococcus aureus; antibiotics; bacteriophages; membrane vesicles.
Copyright © 2019 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

