Pharmacogenetic association between NAT2 gene polymorphisms and isoniazid induced hepatotoxicity: trial sequence meta-analysis as evidence
- PMID: 30509962
- PMCID: PMC6331676
- DOI: 10.1042/BSR20180845
Pharmacogenetic association between NAT2 gene polymorphisms and isoniazid induced hepatotoxicity: trial sequence meta-analysis as evidence
Abstract
Hepatotoxicity is a severe problem generally faced by tuberculosis (TB) patients. It is a well-known adverse reaction due to anti-TB drugs in TB patients undergoing long-term treatment. The studies published previously have explored the connection of N-acetyltransferase 2 (NAT2) gene polymorphisms with isoniazid-induced hepatotoxicity, but the results obtained were inconsistent and inconclusive. A comprehensive trial sequence meta-analysis was conducted employing 12 studies comprising 3613 controls and 933 confirmed TB cases using the databases namely, EMBASE, PubMed (Medline) and Google Scholar till December 2017. A significant association was observed with individuals carrying variant allele at position 481C>T (T vs. C: P = 0.001; OR = 1.278, 95% CI = 1.1100-1.484), at position 590G>A (A vs. G: P = 0.002; OR = 1.421, 95% CI = 1.137-1.776) and at position 857G>A (A vs. G: P = 0.0022; OR = 1.411, 95% CI = 1.052-1.894) to higher risk of hepatotoxicity vis-à-vis wild-type allele. Likewise, the other genetic models of NAT2 gene polymorphisms have also shown increased risk of hepatotoxicity. No evidence of publication bias was observed. These results suggest that genetic variants of NAT2 gene have significant role in isoniazid induced hepatotoxicity. Thus, NAT2 genotyping has the potential to improve the understanding of the drug-enzyme metabolic capacity and help in early predisposition of isoniazid-induced hepatotoxicity.
Keywords: Meta-analysis; NAT2; anti-tuberculosis drug; genetic model; hepatotoxicity.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

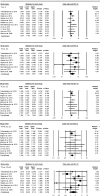
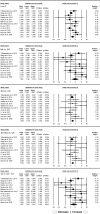

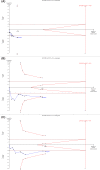
Similar articles
-
Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatotoxicity in Tunisian patients with tuberculosis.Pathol Biol (Paris). 2012 Oct;60(5):324-30. doi: 10.1016/j.patbio.2011.07.001. Pathol Biol (Paris). 2012. PMID: 21856096
-
Association of N-acetyltransferase 2 and cytochrome P450 2E1 gene polymorphisms with antituberculosis drug-induced hepatotoxicity in Western India.J Gastroenterol Hepatol. 2013 Aug;28(8):1368-74. doi: 10.1111/jgh.12194. J Gastroenterol Hepatol. 2013. PMID: 23875638
-
Hepatotoxicity during TB treatment in people with HIV/AIDS related to NAT2 polymorphisms in Pernambuco, Northeast Brazil.Ann Hepatol. 2020 Mar-Apr;19(2):153-160. doi: 10.1016/j.aohep.2019.09.008. Epub 2019 Oct 24. Ann Hepatol. 2020. PMID: 31734174
-
Pharmacogenomic insights into tuberculosis treatment shows the NAT2 genetic variants linked to hepatotoxicity risk: a systematic review and meta-analysis.BMC Genom Data. 2024 Dec 5;25(1):103. doi: 10.1186/s12863-024-01286-y. BMC Genom Data. 2024. PMID: 39639188 Free PMC article.
-
NAT2 polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: a meta-analysis.Int J Tuberc Lung Dis. 2012 May;16(5):589-95. doi: 10.5588/ijtld.11.0377. Epub 2012 Mar 8. Int J Tuberc Lung Dis. 2012. PMID: 22409928 Review.
Cited by
-
Oxidative Stress in Drug-Induced Liver Injury (DILI): From Mechanisms to Biomarkers for Use in Clinical Practice.Antioxidants (Basel). 2021 Mar 5;10(3):390. doi: 10.3390/antiox10030390. Antioxidants (Basel). 2021. PMID: 33807700 Free PMC article. Review.
-
Association of genetic polymorphisms of CYP2E1, NAT2, GST and SLCO1B1 with the risk of anti-tuberculosis drug-induced liver injury: a systematic review and meta-analysis.BMJ Open. 2019 Aug 1;9(8):e027940. doi: 10.1136/bmjopen-2018-027940. BMJ Open. 2019. PMID: 31375612 Free PMC article.
-
N-acetyltransferase Gene Variants Involved in Pediatric Idiosyncratic Drug-Induced Liver Injury.Biomedicines. 2024 Jun 11;12(6):1288. doi: 10.3390/biomedicines12061288. Biomedicines. 2024. PMID: 38927494 Free PMC article.
-
Current Perspective: 3D Spheroid Models Utilizing Human-Based Cells for Investigating Metabolism-Dependent Drug-Induced Liver Injury.Front Med Technol. 2020 Nov 30;2:611913. doi: 10.3389/fmedt.2020.611913. eCollection 2020. Front Med Technol. 2020. PMID: 35047893 Free PMC article. Review.
-
Clinical perspectives of isoniazid-induced liver injury.Liver Res. 2021 Feb 11;5(2):45-52. doi: 10.1016/j.livres.2021.02.001. eCollection 2021 Jun. Liver Res. 2021. PMID: 39959342 Free PMC article. Review.
References
-
- Lauterburg B.H., Smith C.V., Todd E.L. and Mitchell J.R. (1985) Pharmacokinetics of the toxic hydrazino metabolites formed from isoniazid in humans. J. Pharmacol. Exp. Ther. 235, 566–570 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

