Mycoplasma promotes malignant transformation in vivo, and its DnaK, a bacterial chaperone protein, has broad oncogenic properties
- PMID: 30509983
- PMCID: PMC6304983
- DOI: 10.1073/pnas.1815660115
Mycoplasma promotes malignant transformation in vivo, and its DnaK, a bacterial chaperone protein, has broad oncogenic properties
Erratum in
-
Correction for Zella et al., Mycoplasma promotes malignant transformation in vivo, and its DnaK, a bacterial chaperon protein, has broad oncogenic properties.Proc Natl Acad Sci U S A. 2019 Jan 15;116(3):1069. doi: 10.1073/pnas.1821037116. Epub 2019 Jan 7. Proc Natl Acad Sci U S A. 2019. PMID: 30617068 Free PMC article. No abstract available.
Abstract
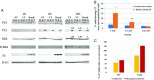
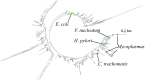
We isolated a strain of human mycoplasma that promotes lymphomagenesis in SCID mice, pointing to a p53-dependent mechanism similar to lymphomagenesis in uninfected p53-/- SCID mice. Additionally, mycoplasma infection in vitro reduces p53 activity. Immunoprecipitation of p53 in mycoplasma-infected cells identified several mycoplasma proteins, including DnaK, a member of the Hsp70 chaperon family. We focused on DnaK because of its ability to interact with proteins. We demonstrate that mycoplasma DnaK interacts with and reduces the activities of human proteins involved in critical cellular pathways, including DNA-PK and PARP1, which are required for efficient DNA repair, and binds to USP10 (a key p53 regulator), impairing p53-dependent anticancer functions. This also reduced the efficacy of anticancer drugs that depend on p53 to exert their effect. mycoplasma was detected early in the infected mice, but only low copy numbers of mycoplasma DnaK DNA sequences were found in some primary and secondary tumors, pointing toward a hit-and-run/hide mechanism of transformation. Uninfected bystander cells took up exogenous DnaK, suggesting a possible paracrine function in promoting malignant transformation, over and above cells infected with the mycoplasma. Phylogenetic amino acid analysis shows that other bacteria associated with human cancers have similar DnaKs, consistent with a common mechanism of cellular transformation mediated through disruption of DNA-repair mechanisms, as well as p53 dysregulation, that also results in cancer-drug resistance. This suggests that the oncogenic properties of certain bacteria are DnaK-mediated.
Keywords: DNA repair; DnaK; cancer; mycoplasma; p53.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer. 2018;18:359–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

