Constitutive boost of a K+ channel via inherent bilayer tension and a unique tension-dependent modality
- PMID: 30509986
- PMCID: PMC6304998
- DOI: 10.1073/pnas.1812282115
Constitutive boost of a K+ channel via inherent bilayer tension and a unique tension-dependent modality
Abstract
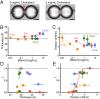
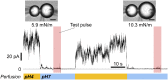
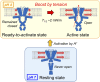
Molecular mechanisms underlying channel-membrane interplay have been extensively studied. Cholesterol, as a major component of the cell membrane, participates either in specific binding to channels or via modification of membrane physical features. Here, we examined the action of various sterols (cholesterol, epicholesterol, etc.) on a prototypical potassium channel (KcsA). Single-channel current recordings of the KcsA channel were performed in a water-in-oil droplet bilayer (contact bubble bilayer) with a mixed phospholipid composition (azolectin). Upon membrane perfusion of sterols, the activated gate at acidic pH closed immediately, irrespective of the sterol species. During perfusion, we found that the contacting bubbles changed their shapes, indicating alterations in membrane physical features. Absolute bilayer tension was measured according to the principle of surface chemistry, and inherent bilayer tension was ∼5 mN/m. All tested sterols decreased the tension, and the nonspecific sterol action to the channel was likely mediated by the bilayer tension. Purely mechanical manipulation that reduced bilayer tension also closed the gate, whereas the resting channel at neutral pH never activated upon increased tension. Thus, rather than conventional stretch activation, the channel, once ready to activate by acidic pH, changes the open probability through the action of bilayer tension. This constitutes a channel regulating modality by two successive stimuli. In the contact bubble bilayer, inherent bilayer tension was high, and the channel remained boosted. In the cell membrane, resting tension is low, and it is anticipated that the ready-to-activate channel remains closed until bilayer tension reaches a few millinewton/meter during physiological and pathological cellular activities.
Keywords: KcsA channel; bilayer tension; contact bubble bilayer; single-channel current; stretch-activated channel.
Figures


References
-
- Tillman TS, Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys. 2003;38:161–190. - PubMed
-
- Nicolson GL. The fluid-mosaic model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta. 2014;1838:1451–1466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

