Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson's disease and DLB
- PMID: 30509990
- PMCID: PMC6304960
- DOI: 10.1073/pnas.1813365115
Synergistic neuroprotection by coffee components eicosanoyl-5-hydroxytryptamide and caffeine in models of Parkinson's disease and DLB
Abstract
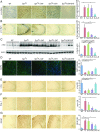
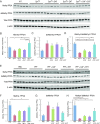
Hyperphosphorylated α-synuclein in Lewy bodies and Lewy neurites is a characteristic neuropathological feature of Parkinson's disease (PD) and Dementia with Lewy bodies (DLB). The catalytic subunit of the specific phosphatase, protein phosphatase 2A (PP2A) that dephosphorylates α-synuclein, is hypomethylated in these brains, thereby impeding the assembly of the active trimeric holoenzyme and reducing phosphatase activity. This phosphatase deficiency contributes to the accumulation of hyperphosphorylated α-synuclein, which tends to fibrillize more than unmodified α-synuclein. Eicosanoyl-5-hydroxytryptamide (EHT), a fatty acid derivative of serotonin found in coffee, inhibits the PP2A methylesterase so as to maintain PP2A in a highly active methylated state and mitigates the phenotype of α-synuclein transgenic (SynTg) mice. Considering epidemiologic and experimental evidence suggesting protective effects of caffeine in PD, we sought, in the present study, to test whether there is synergy between EHT and caffeine in models of α-synucleinopathy. Coadministration of these two compounds orally for 6 mo at doses that were individually ineffective in SynTg mice and in a striatal α-synuclein preformed fibril inoculation model resulted in reduced accumulation of phosphorylated α-synuclein, preserved neuronal integrity and function, diminished neuroinflammation, and improved behavioral performance. These indices were associated with increased levels of methylated PP2A in brain tissue. A similar profile of greater PP2A methylation and cytoprotection was found in SH-SY5Y cells cotreated with EHT and caffeine, but not with each compound alone. These findings suggest that these two components of coffee have synergistic effects in protecting the brain against α-synuclein-mediated toxicity through maintenance of PP2A in an active state.
Keywords: Dementia with Lewy bodies; Parkinson’s disease; neuroprotection; phosphorylation; α-synuclein.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: J.B.S. has a financial interest in Signum Biosciences, which is developing PP2A phosphatase enhancing agents. M.V. is employed by Signum Biosciences. J.B.S. and M.M.M. are inventors of a patent application relevant to this work.
Figures
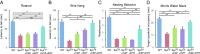
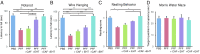


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

