Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study
- PMID: 30510079
- PMCID: PMC6368201
- DOI: 10.1182/blood-2018-09-876805
Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study
Abstract
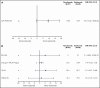
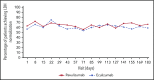
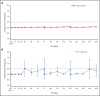
Ravulizumab, a new complement component C5 inhibitor administered every 8 weeks, was noninferior to eculizumab administered every 2 weeks in complement-inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria (PNH). This study assessed noninferiority of ravulizumab to eculizumab in clinically stable PNH patients during previous eculizumab therapy. In this phase 3, open-label, multicenter study, 195 PNH patients on labeled-dose (900 mg every 2 weeks) eculizumab for >6 months were randomly assigned 1:1 to switch to ravulizumab (n = 97) or continue eculizumab (n = 98). Primary efficacy end point was percentage change in lactate dehydrogenase (LDH) from baseline to day 183. Key secondary end points included proportion of patients with breakthrough hemolysis, change in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score, transfusion avoidance, and stabilized hemoglobin. In 191 patients completing 183 days of treatment, ravulizumab was noninferior to eculizumab (P inf < .0006 for all end points), including percentage change in LDH (difference, 9.21% [95% confidence interval (CI), -0.42 to 18.84], P = .058 for superiority), breakthrough hemolysis (difference, 5.1 [95% CI, -8.89 to 18.99]), change in FACIT-Fatigue score (difference, 1.47 [95% CI, -0.21 to 3.15]), transfusion avoidance (difference, 5.5 [95% CI, -4.27 to 15.68]), and stabilized hemoglobin (difference, 1.4 [95% CI, -10.41 to 13.31]). The most frequently reported adverse event was headache (26.8%, ravulizumab; 17.3%, eculizumab). No meningococcal infections or discontinuations due to adverse events occurred. Patients with PNH may be safely and effectively switched from labeled-dose eculizumab administered every 2 weeks to ravulizumab administered every 8 weeks. This trial was funded by Alexion Pharmaceuticals, Inc., and is registered at www.clinicaltrials.gov as #NCT03056040.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.G.K., A.H., R.W., F.A.G.-F., A.G., E.O.G., A.R., and S.N. have received honoraria and consulting fees from Alexion Pharmaceuticals, Inc. S.T.R., L.S., A.I.D., and S.O. are employees and stockholders of Alexion Pharmaceuticals, Inc. J.W.L. has received honoraria, consulting fees, and research support (to Seoul St. Mary’s Hospital) from Alexion Pharmaceuticals, Inc. E.B. is a former employee and stockholder of Alexion Pharmaceuticals, Inc. J.S. has received consultancies, honoraria, and advisory board membership for Alexion Pharmaceuticals, Inc. A.R. has received consultancies and honoraria from Alexion, Roche, and Novartis and research funding from Alexion and Roche. R.P.d.L. has received consultancies, honoraria, and research funding from Alexion, Novartis, and Pfizer and research funding from Amgen. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Ravulizumab: a complementary option for PNH.Blood. 2019 Feb 7;133(6):503-504. doi: 10.1182/blood-2018-12-891499. Blood. 2019. PMID: 30733201 No abstract available.
References
-
- Hillmen P, Young NS, Schubert J, et al. . The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233-1243. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

