Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study
- PMID: 30510080
- PMCID: PMC6367644
- DOI: 10.1182/blood-2018-09-876136
Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study
Abstract
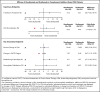
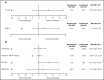
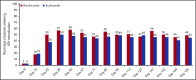
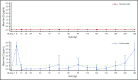
Ravulizumab (ALXN1210), a new complement C5 inhibitor, provides immediate, complete, and sustained C5 inhibition. This phase 3, open-label study assessed the noninferiority of ravulizumab to eculizumab in complement inhibitor-naive adults with paroxysmal nocturnal hemoglobinuria (PNH). Patients with lactate dehydrogenase (LDH) ≥1.5 times the upper limit of normal and at least 1 PNH symptom were randomized 1:1 to receive ravulizumab or eculizumab for 183 days (N = 246). Coprimary efficacy end points were proportion of patients remaining transfusion-free and LDH normalization. Secondary end points were percent change from baseline in LDH, change from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score, proportion of patients with breakthrough hemolysis, stabilized hemoglobin, and change in serum free C5. Ravulizumab was noninferior to eculizumab for both coprimary and all key secondary end points (Pinf < .0001): transfusion avoidance (73.6% vs 66.1%; difference of 6.8% [95% confidence interval (CI), -4.66, 18.14]), LDH normalization (53.6% vs 49.4%; odds ratio, 1.19 [0.80, 1.77]), percent reduction in LDH (-76.8% vs -76.0%; difference [95% CI], -0.83% [-5.21, 3.56]), change in FACIT-Fatigue score (7.07 vs 6.40; difference [95% CI], 0.67 [-1.21, 2.55]), breakthrough hemolysis (4.0% vs 10.7%; difference [95% CI], -6.7% [-14.21, 0.18]), and stabilized hemoglobin (68.0% vs 64.5%; difference [95% CI], 2.9 [-8.80, 14.64]). The safety and tolerability of ravulizumab and eculizumab were similar; no meningococcal infections occurred. In conclusion, ravulizumab given every 8 weeks achieved noninferiority compared with eculizumab given every 2 weeks for all efficacy end points, with a similar safety profile. This trial was registered at www.clinicaltrials.gov as #NCT02946463.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.W.L. has received honoraria, consulting fees, and research support (to Seoul St. Mary’s Hospital) from Alexion Pharmaceuticals, Inc. F.S.d.F. has received honoraria and research support (to St. Louis Hospital) from Alexion Pharmaceuticals, Inc. V. Pessoa and S.G. have received research support from Alexion Pharmaceuticals, Inc. W.F. has received honoraria from Alexion Pharmaceuticals, Inc, and Novartis. V. Ptushkin has received honoraria from Alexion Pharmaceuticals, Inc. S.T.R., L.V., L.S., R.A., and R.P. are employees and stockholders of Alexion Pharmaceuticals, Inc. H.S. has received honoraria and research support (all to University of Ulm) from Alexion Pharmaceuticals, Inc. A.H. has received honoraria and consulting fees from Alexion Pharmaceuticals, Inc. L.W.L.L. declares no competing financial interests.
Figures
Comment in
-
Ravulizumab: a complementary option for PNH.Blood. 2019 Feb 7;133(6):503-504. doi: 10.1182/blood-2018-12-891499. Blood. 2019. PMID: 30733201 No abstract available.
Similar articles
-
Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study.Blood. 2019 Feb 7;133(6):540-549. doi: 10.1182/blood-2018-09-876805. Epub 2018 Dec 3. Blood. 2019. PMID: 30510079 Free PMC article. Clinical Trial.
-
One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab.Eur J Haematol. 2021 Mar;106(3):389-397. doi: 10.1111/ejh.13564. Epub 2021 Jan 3. Eur J Haematol. 2021. PMID: 33301613 Free PMC article. Clinical Trial.
-
One-year efficacy and safety of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria naïve to complement inhibitor therapy: open-label extension of a randomized study.Ther Adv Hematol. 2020 Oct 24;11:2040620720966137. doi: 10.1177/2040620720966137. eCollection 2020. Ther Adv Hematol. 2020. PMID: 33178408 Free PMC article.
-
Ravulizumab for the treatment of paroxysmal nocturnal hemoglobinuria.Expert Opin Biol Ther. 2020 Mar;20(3):227-237. doi: 10.1080/14712598.2020.1725468. Epub 2020 Feb 14. Expert Opin Biol Ther. 2020. PMID: 32011183 Review.
-
Anti-complement Treatment for Paroxysmal Nocturnal Hemoglobinuria: Time for Proximal Complement Inhibition? A Position Paper From the SAAWP of the EBMT.Front Immunol. 2019 Jun 14;10:1157. doi: 10.3389/fimmu.2019.01157. eCollection 2019. Front Immunol. 2019. PMID: 31258525 Free PMC article. Review.
Cited by
-
Successful management of unstable angina in a ravulizumab-treated patient with paroxysmal nocturnal hemoglobinuria.Fukushima J Med Sci. 2022 Dec 21;68(3):175-178. doi: 10.5387/fms.2022-16. Epub 2022 Sep 22. Fukushima J Med Sci. 2022. PMID: 36130907 Free PMC article.
-
Treatment and Relapse Prevention of Typical and Atypical Optic Neuritis.Int J Mol Sci. 2022 Aug 29;23(17):9769. doi: 10.3390/ijms23179769. Int J Mol Sci. 2022. PMID: 36077167 Free PMC article. Review.
-
Novel insights into the treatment of complement-mediated hemolytic anemias.Ther Adv Hematol. 2019 Sep 9;10:2040620719873321. doi: 10.1177/2040620719873321. eCollection 2019. Ther Adv Hematol. 2019. PMID: 31523413 Free PMC article. Review.
-
Complementopathies and precision medicine.J Clin Invest. 2020 May 1;130(5):2152-2163. doi: 10.1172/JCI136094. J Clin Invest. 2020. PMID: 32310222 Free PMC article. Review.
-
Eculizumab for paroxysmal nocturnal haemoglobinuria: catastrophic health expenditure in Nepalese patients.Orphanet J Rare Dis. 2023 Jun 30;18(1):172. doi: 10.1186/s13023-023-02779-2. Orphanet J Rare Dis. 2023. PMID: 37391775 Free PMC article.
References
-
- Hillmen P, Young NS, Schubert J, et al. . The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233-1243. - PubMed
-
- Brodsky RA, Young NS, Antonioli E, et al. . Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840-1847. - PubMed
-
- Hillmen P, Muus P, Dührsen U, et al. . Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123-4128. - PubMed
-
- US Food and Drug Administration. Soliris (eculizumab); prescribing information. Boston, MA: Alexion Pharmaceuticals, Inc; 2018.
-
- European Medicines Agency. Soliris (eculizumab); summary of product characteristics. Paris, France: Alexion Europe SAS; 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

