Molecular remission and response patterns in patients with mutant- IDH2 acute myeloid leukemia treated with enasidenib
- PMID: 30510081
- PMCID: PMC6384189
- DOI: 10.1182/blood-2018-08-869008
Molecular remission and response patterns in patients with mutant- IDH2 acute myeloid leukemia treated with enasidenib
Abstract
Approximately 8% to 19% of patients with acute myeloid leukemia (AML) have isocitrate dehydrogenase-2 (IDH2) mutations, which occur at active site arginine residues R140 and R172. IDH2 mutations produce an oncometabolite, 2-hydroxyglutarate (2-HG), which leads to DNA and histone hypermethylation and impaired hematopoietic differentiation. Enasidenib is an oral inhibitor of mutant-IDH2 proteins. This first-in-human phase 1/2 study evaluated enasidenib doses of 50 to 650 mg/d, administered in continuous 28-day cycles, in patients with mutant-IDH2 hematologic malignancies. Overall, 214 of 345 patients (62%) with relapsed or refractory (R/R) AML received enasidenib, 100 mg/d. Median age was 68 years. Forty-two patients (19.6%) attained complete remission (CR), 19 patients (10.3%) proceeded to an allogeneic bone marrow transplant, and the overall response rate was 38.8% (95% confidence interval [CI], 32.2-45.7). Median overall survival was 8.8 months (95% CI, 7.7-9.6). Response and survival were comparable among patients with IDH2-R140 or IDH2-R172 mutations. Response rates were similar among patients who, at study entry, were in relapse (37.7%) or were refractory to intensive (37.5%) or nonintensive (43.2%) therapies. Sixty-six (43.1%) red blood cell transfusion-dependent and 53 (40.2%) platelet transfusion-dependent patients achieved transfusion independence. The magnitude of 2-HG reduction on study was associated with CR in IDH2-R172 patients. Clearance of mutant-IDH2 clones was also associated with achievement of CR. Among all 345 patients, the most common grade 3 or 4 treatment-related adverse events were hyperbilirubinemia (10%), thrombocytopenia (7%), and IDH differentiation syndrome (6%). Enasidenib was well tolerated and induced molecular remissions and hematologic responses in patients with AML for whom prior treatments had failed. The study is registered at www.clinicaltrials.gov as #NCT01915498.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: E.M.S. has served on advisory boards for Astellas Pharma, Daiichi, Bayer, Novartis, Pfizer, Agios, and Celgene and has received research funding from Agios, Celgene, Syros, GlaxoSmithKline, and Bayer. C.D.D. is a consultant for AbbVie, Agios, and Celgene, and has received honoraria from Bayer, Jazz, Karyopharm, MedImmune, and Syros as an advisory board member. A.T.F. has served as a consultant for Takeda, Seattle Genetics, and Celgene and has served on advisory boards for Agios, Jazz Pharmaceuticals, Boehringer Ingelheim, and Astellas Pharma. D.A.P. has received honoraria from Agios and Pfizer and has served on advisory boards for Pfizer, Celyad, Agios, Celgene, AbbVie, Argenx, and Curis. R.M.S. has served as a consultant for AbbVie, Agios, Amgen, Arog Pharmaceuticals, Astellas Pharma, Celator Pharmaceuticals, Celgene, Cornerstone, FUJIFILM Pharmaceuticals, Janssen, Jazz Pharmaceuticals, Juno Therapeutics, Karyopharm, Merck, Novartis, Ono Pharmaceutical, Orsenix, Pfizer, Roche, and Sumitomo; has received research funding from Agios, Arog Pharmaceuticals, and Novartis; has served on advisory boards for Actinium; has served on data safety monitoring boards for argenx and Celgene; and has served on a steering committee for Celgene. J.K.A. has served on advisory boards for Syros, Astellas Pharma, Janssen, Novartis, Seattle Genetics, Spectrum, ARIAD Pharmaceuticals, Bristol-Myers-Squibb, Immune Pharmaceuticals, Agios, and Celgene, serves on a data safety and monitoring committee for GlycoMimetics; her institution has received research funding from MethylGene, Boehringer Ingelheim, Astellas Pharma, Agios, Bristol-Myers-Squibb, CSL Limited, Cyclacel Pharmaceuticals, Epizyme, Genentech, Pfizer, BioLineRX, Talon Therapeutics, Celgene, Amphivena, and FUJIFILM Pharmaceuticals. G.J.R. has served as a consultant for Celgene, Agios, AbbVie, Amgen, Amphivena, argenx, Array BioPharma, Astex, AstraZeneca, Bayer, Celator Pharmaceuticals, Celltrion, Clovis Oncology, CTI BioPharma, Eisai, Genoptix, Immune Pharmaceuticals, Janssen, Jazz Pharmaceuticals, Juno Therapeutics, MEI Pharma, MedImmune, Novartis, Onconova, Orsenix, Pfizer, Roche/Genentech, Sunesis, and Sandoz and has received research support from Cellectis. R.C. has received research funding from Agios. I.W.F. has received research funding from Acerta, Agios, BeiGene, Calithera, Celgene, Constellation, Curis, Forma, Forty Seven, Genentech, Gilead, Incyte, Janssen, Merck, Novartis, Pfizer, Pharmacyclics, Seattle Genetics, Takeda, TG Therapeutics, and Verastem. M.A.S. has served on advisory boards for Celgene. A.S.S. has served on advisory boards for Stemline Therapeutics and has served on speakers’ bureaus for Amgen and Celgene. H.M.K. has received research funding from Amgen, ARIAD Pharmaceuticals, Astex, Bristol-Myers-Squibb, Novartis, and Pfizer. R.L.L. is on the supervisory board of QIAGEN and is a scientific advisor for Loxo, Imago, C4 Therapeutics, and Isoplexis, which each include an equity interest; receives research support from and has consulted for Celgene and Roche; has received research support from Prelude Therapeutics; has acted as a consultant for Incyte, Novartis, Morphosys, and Janssen; and has received honoraria from Lilly and Amgen for invited lectures and from Gilead for grant reviews. K.J.M., A.T., Q.X., T.L., and A.R. are employed by and own stock in Celgene Corporation. J.V. and I.G. were employed by Celgene Corporation at the time of the study. K.E.Y., B.W., and E.C.A. are employed by and own stock in Agios. M.S.T. has received research funding from Arog Pharmaceuticals, Bioline, Cellerant Therapeutics, ADC Therapeutics, Celgene, Daiichi Sankyo, and Orsenix. S.d.B. has served on advisory boards for Agios, Celgene, Pfizer, Novartis, Servier, Pierre Fabre, Bayer, Seattle Genetics, Carthagenetics, and FORMA Therapeutics and has received research funding from Agios. The remaining authors declare no competing financial interests.
Figures

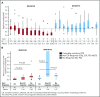
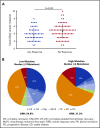
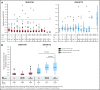
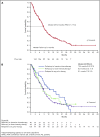
Comment in
-
IDH2 inhibition: another piece to the puzzle.Blood. 2019 Feb 14;133(7):625-626. doi: 10.1182/blood-2018-12-891481. Blood. 2019. PMID: 30765493 No abstract available.
References
-
- Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol. 2017;18(3):17. - PubMed
-
- Karanes C, Kopecky KJ, Head DR, et al. . A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res. 1999;23(9):787-794. - PubMed
-
- Herzig RH, Lazarus HM, Wolff SN, Phillips GL, Herzig GP. High-dose cytosine arabinoside therapy with and without anthracycline antibiotics for remission reinduction of acute nonlymphoblastic leukemia. J Clin Oncol. 1985;3(7):992-997. - PubMed
-
- Roboz GJ, Rosenblat T, Arellano M, et al. . International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol. 2014;32(18):1919-1926. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

