Downregulation of the Central Noradrenergic System by Toxoplasma gondii Infection
- PMID: 30510101
- PMCID: PMC6346129
- DOI: 10.1128/IAI.00789-18
Downregulation of the Central Noradrenergic System by Toxoplasma gondii Infection
Abstract
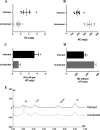
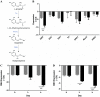
Toxoplasma gondii is associated with physiological effects in the host. Dysregulation of catecholamines in the central nervous system has previously been observed in chronically infected animals. In the study described here, the noradrenergic system was found to be suppressed with decreased levels of norepinephrine (NE) in brains of infected animals and in infected human and rat neural cells in vitro The mechanism responsible for the NE suppression was found to be downregulation of dopamine β-hydroxylase (DBH) gene expression, encoding the enzyme that synthesizes norepinephrine from dopamine, with downregulation observed in vitro and in infected brain tissue, particularly in the dorsal locus coeruleus/pons region. The downregulation was sex specific, with males expressing reduced DBH mRNA levels whereas females were unchanged. Rather, DBH expression correlated with estrogen receptor in the female rat brains for this estrogen-regulated gene. DBH silencing was not a general response of neurons to infection, as human cytomegalovirus did not downregulate DBH expression. The noradrenergic-linked behaviors of sociability and arousal were altered in chronically infected animals, with a high correlation between DBH expression and infection intensity. A decrease in DBH expression in noradrenergic neurons can elevate dopamine levels, which provides a possible explanation for mixed observations of changes in this neurotransmitter with infection. Decreased NE is consistent with the loss of coordination and motor impairments associated with toxoplasmosis. Further, the altered norepinephrine synthesis observed here may, in part, explain behavioral effects of infection and associations with mental illness.
Keywords: apicomplexan parasites; behavior; bradyzoite; host-pathogen interactions; intracellular parasites; norepinephrine; protozoa.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- Wastling J, Heap S, Ferguson D. 2000. Toxoplasma gondii–keeping our guests under control. Biologist (Lond) 47:234–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

