Engineering of a GLP-1 analogue peptide/anti-PCSK9 antibody fusion for type 2 diabetes treatment
- PMID: 30510163
- PMCID: PMC6277417
- DOI: 10.1038/s41598-018-35869-4
Engineering of a GLP-1 analogue peptide/anti-PCSK9 antibody fusion for type 2 diabetes treatment
Abstract
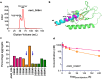
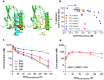
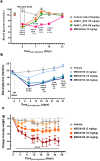
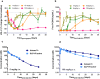
Type 2 diabetes (T2D) is a complex and progressive disease requiring polypharmacy to manage hyperglycaemia and cardiovascular risk factors. However, most patients do not achieve combined treatment goals. To address this therapeutic gap, we have developed MEDI4166, a novel glucagon-like peptide-1 (GLP-1) receptor agonist peptide fused to a proprotein convertase subtilisin/kexin type 9 (PCSK9) neutralising antibody that allows for glycaemic control and low-density lipoprotein cholesterol (LDL-C) lowering in a single molecule. The fusion has been engineered to deliver sustained peptide activity in vivo in combination with reduced potency, to manage GLP-1 driven adverse effects at high dose, and a favourable manufacturability profile. MEDI4166 showed robust and sustained LDL-C lowering in cynomolgus monkeys and exhibited the anticipated GLP-1 effects in T2D mouse models. We believe MEDI4166 is a novel molecule combining long acting agonist peptide and neutralising antibody activities to deliver a unique pharmacology profile for the management of T2D.
Conflict of interest statement
All authors, with the exception of A.J.C., A.K., D.F., E.R., K.D., L.J. and T.B.L., are employees of the AstraZeneca group of companies. A.J.C., A.K., D.F., E.R., K.D., L.J. and T.B.L. are former employees of MedImmune, a company of the AstraZeneca group. All authors may have stocks/stock options in AstraZeneca.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

