Angiotensin II Attenuates the Bioactivities of Human Endothelial Progenitor Cells via Downregulation of β 2-Adrenergic Receptor
- PMID: 30510587
- PMCID: PMC6231359
- DOI: 10.1155/2018/7453161
Angiotensin II Attenuates the Bioactivities of Human Endothelial Progenitor Cells via Downregulation of β 2-Adrenergic Receptor
Abstract
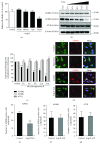
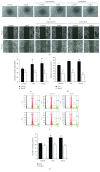
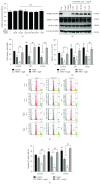
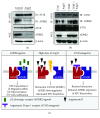
Cross talks between the renin-angiotensin system (RAS), sympathetic nervous system, and vascular homeostasis are tightly coordinated in hypertension. Angiotensin II (Ang II), a key factor in RAS, when abnormally activated, affects the number and bioactivity of circulating human endothelial progenitor cells (hEPCs) in hypertensive patients. In this study, we investigated how the augmentation of Ang II regulates adrenergic receptor-mediated signaling and angiogenic bioactivities of hEPCs. Interestingly, the short-term treatment of hEPCs with Ang II drastically attenuated the expression of beta-2 adrenergic receptor (ADRB2), but did not alter the expression of beta-1 adrenergic receptor (ADRB1) and Ang II type 1 receptor (AT1R). EPC functional assay clearly demonstrated that the treatment with ADRB2 agonists significantly increased EPC bioactivities including cell proliferation, migration, and tube formation abilities. However, EPC bioactivities were decreased dramatically when treated with Ang II. Importantly, the attenuation of EPC bioactivities by Ang II was restored by treatment with an AT1R antagonist (telmisartan; TERT). We found that AT1R binds to ADRB2 in physiological conditions, but this binding is significantly decreased in the presence of Ang II. Furthermore, TERT, an Ang II-AT1R interaction blocker, restored the interaction between AT1R and ADRB2, suggesting that Ang II might induce the dysfunction of EPCs via downregulation of ADRB2, and an AT1R blocker could prevent Ang II-mediated ADRB2 depletion in EPCs. Taken together, our report provides novel insights into potential therapeutic approaches for hypertension-related cardiovascular diseases.
Figures
References
-
- de Cavanagh E. M. V., González S. A., Inserra F., et al. Sympathetic predominance is associated with impaired endothelial progenitor cells and tunneling nanotubes in controlled-hypertensive patients. American Journal of Physiology. Heart and Circulatory Physiology. 2014;307(2):H207–H215. doi: 10.1152/ajpheart.00955.2013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

