Weighing In on mTOR Complex 2 Signaling: The Expanding Role in Cell Metabolism
- PMID: 30510625
- PMCID: PMC6232796
- DOI: 10.1155/2018/7838647
Weighing In on mTOR Complex 2 Signaling: The Expanding Role in Cell Metabolism
Abstract
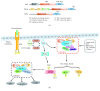
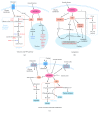
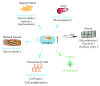
In all eukaryotes, the mechanistic target of rapamycin (mTOR) signaling emerges as a master regulator of homeostasis, which integrates environmental inputs, including nutrients, energy, and growth factors, to regulate many fundamental cellular processes such as cell growth and metabolism. mTOR signaling functions through two structurally and functionally distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which correspond to two major branches of signal output. While mTORC1 is well characterized for its structure, regulation, and function in the last decade, information of mTORC2 signaling is only rapidly expanding in recent years, from structural biology, signaling network, to functional impact. Here we review the recent advances in many aspects of the mTORC2 signaling, with particular focus on its involvement in the control of cell metabolism and its physiological implications in metabolic diseases and aging.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

