A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus
- PMID: 30510843
- PMCID: PMC6261870
A review of clinical trials: mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus
Abstract
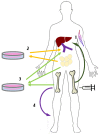
Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are widely prevalent metabolic diseases with differing pathologies. T1DM manifests due to autoimmune destruction of the pancreatic beta cells, resulting in a diminished secretion of insulin. T2DM originates from a state of insulin resistance, resulting in hyperglycemia and reduction in beta cell mass. Both diseases can cause severe health consequences. Despite the globally increasing prevalence of both T1DM and T2DM there remains to be a medically defined cure for either of these diseases. Recently, mesenchymal stem cells (MSCs) have been proposed as a possible curative treatment method. In this review, we explain the molecular mechanisms underlying MSCs and their potential ability to treat T1DM and T2DM. We describe the capability of MSCs to differentiate into insulin-producing cells and regenerate pancreatic beta cells, as well as assess their role in modulating the immune system. Lastly, we evaluate the current literature focusing on the clinical application of MSC transplantation in T1DM and T2DM. Despite the favorable results, study designs and analyses cast doubt on the effectiveness of MSCs for the management of T1DM. Conversely, the positive metabolic effects consistently demonstrated in the literature offer hope for MSCs as a treatment for T2DM, at least in the short-term.
Keywords: Diabetes mellitus; clinical application; clinical trials; insulin sensitivity; mesenchymal stem cells; stem cell therapy; transplantation; type I diabetes; type II diabetes.
Conflict of interest statement
None.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
