Breaking Bad: How Viruses Subvert the Cell Cycle
- PMID: 30510918
- PMCID: PMC6252338
- DOI: 10.3389/fcimb.2018.00396
Breaking Bad: How Viruses Subvert the Cell Cycle
Abstract
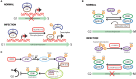
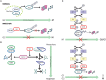
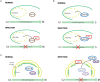
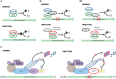
Interactions between the host and viruses during the course of their co-evolution have not only shaped cellular function and the immune system, but also the counter measures employed by viruses. Relatively small genomes and high replication rates allow viruses to accumulate mutations and continuously present the host with new challenges. It is therefore, no surprise that they either escape detection or modulate host physiology, often by redirecting normal cellular pathways to their own advantage. Viruses utilize a diverse array of strategies and molecular targets to subvert host cellular processes, while evading detection. These include cell-cycle regulation, major histocompatibility complex-restricted antigen presentation, intracellular protein transport, apoptosis, cytokine-mediated signaling, and humoral immune responses. Moreover, viruses routinely manipulate the host cell cycle to create a favorable environment for replication, largely by deregulating cell cycle checkpoints. This review focuses on our current understanding of the molecular aspects of cell cycle regulation that are often targeted by viruses. Further study of their interactions should provide fundamental insights into cell cycle regulation and improve our ability to exploit these viruses.
Keywords: cell cycle; checkpoint; degradation; host-pathogen interactions; infection; life cycle; phosphorylation; viruses.
Figures

References
-
- Ahn J. Y., Schwarz J. K., Piwnica-Worms H., Canman C. E. (2000). Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res 60, 5934–5936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

