Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a Phase II trial (ESLC01 study)
- PMID: 30510922
- PMCID: PMC6250115
- DOI: 10.2147/JHC.S169285
Sorafenib plus tegafur-uracil (UFT) versus sorafenib as first line systemic treatment for patients with advanced stage HCC: a Phase II trial (ESLC01 study)
Abstract
Background: Phase II trials found that tegafur-uracil (UFT) is an effective drug in hepatocellular carcinoma (HCC), while preclinical data suggested that its combination with sorafenib may have a promising activity. Our Phase II randomized trial aimed to evaluate efficacy and tolerability of sorafenib plus UFT vs sorafenib in advanced HCC.
Methods: Patients with advanced HCC, with no prior systemic therapy, were randomized to receive either UFT at 125 mg/m2 twice daily for 4 out of 5 weeks plus sorafenib at 400 mg twice daily (arm 1) or single agent sorafenib at 400 mg twice daily (arm 2). Primary end point was time to progression (TTP).
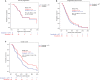
Results: Between March 2012 and March 2014, 76 eligible patients - out of 143 preplanned - were randomized. The study was terminated early because of futility. This is the final analysis of the study, after a median follow-up of 10.2 months and death of 86% of randomized patients (n=64). Median TTP was 7.5 months and 8.2 months in arms 1 and 2 respectively (HR: 1.07; 95% CI, 0.52-2.22; P=0.855), while the median overall survival was 8.2 months and 10.5 months respectively (HR: 1.58; 95% CI: 0.90-2.76, P=0.112). Nine patients (25%) in the combination arm discontinued treatment because of toxicity vs eight patients (21.1%) in the sorafenib monotherapy arm (P=0.899).
Conclusion: In patients with advanced HCC, adding UFT to sorafenib is feasible, but it did not improve efficacy outcome over sorafenib monotherapy.
Keywords: Egypt; advanced hepatocellular carcinoma; sorafenib; tegafur/uracil.
Conflict of interest statement
Disclosure IW received grants/research supports from: Abbvie, Gilead, Janssen, Pharco, Mylan, Onxio and participated in company sponsored speaker’s bureau of Abbvie, Eva-Pharma, Gilead, Janssen, Marcyrl, Mylan, and Roche. The authors report no other conflicts of interest in this work.
Figures

References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. - PubMed
-
- American Cancer Society A, Ga Cancer Facts and Figures, 2017. 2017. [Accessed April 2, 2017]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- Chlebowski RT, Brzechwa-Adjukiewicz A, Cowden A, Block JB, Tong M, Chan KK. Doxorubicin (75 mg/m2) for hepatocellular carcinoma: clinical and pharmacokinetic results. Cancer Treat Rep. 1984;68(3):487–491. - PubMed
LinkOut - more resources
Full Text Sources

