Disruption of FOXP3-EZH2 Interaction Represents a Pathobiological Mechanism in Intestinal Inflammation
- PMID: 30510991
- PMCID: PMC6260395
- DOI: 10.1016/j.jcmgh.2018.08.009
Disruption of FOXP3-EZH2 Interaction Represents a Pathobiological Mechanism in Intestinal Inflammation
Abstract
Background & aims: Forkhead box protein 3 (FOXP3)+ regulatory T cell (Treg) dysfunction is associated with autoimmune diseases; however, the mechanisms responsible for inflammatory bowel disease pathophysiology are poorly understood. Here, we tested the hypothesis that a physical interaction between transcription factor FOXP3 and the epigenetic enzyme enhancer of zeste homolog 2 (EZH2) is essential for gene co-repressive function.
Methods: Human FOXP3 mutations clinically relevant to intestinal inflammation were generated by site-directed mutagenesis. T lymphocytes were isolated from mice, human blood, and lamina propria of Crohn's disease (CD) patients and non-CD controls. We performed proximity ligation or a co-immunoprecipitation assay in FOXP3-mutant+, interleukin 6 (IL6)-treated or CD-CD4+ T cells to assess FOXP3-EZH2 protein interaction. We studied IL2 promoter activity and chromatin state of the interferon γ locus via luciferase reporter and chromatin-immunoprecipitation assays, respectively, in cells expressing FOXP3 mutants.
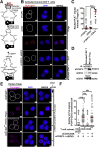
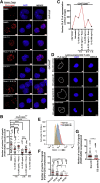
Results: EZH2 binding was abrogated by inflammatory bowel disease-associated FOXP3 cysteine 232 (C232) mutation. The C232 mutant showed impaired repression of IL2 and diminished EZH2-mediated trimethylation of histone 3 at lysine 27 on interferon γ, indicative of compromised Treg physiologic function. Generalizing this mechanism, IL6 impaired FOXP3-EZH2 interaction. IL6-induced effects were reversed by Janus kinase 1/2 inhibition. In lamina propria-derived CD4+T cells from CD patients, we observed decreased FOXP3-EZH2 interaction.
Conclusions: FOXP3-C232 mutation disrupts EZH2 recruitment and gene co-repressive function. The proinflammatory cytokine IL6 abrogates FOXP3-EZH2 interaction. Studies in lesion-derived CD4+ T cells have shown that reduced FOXP3-EZH2 interaction is a molecular feature of CD patients. Destabilized FOXP3-EZH2 protein interaction via diverse mechanisms and consequent Treg abnormality may drive gastrointestinal inflammation.
Keywords: C232, cysteine 232; CD, Crohn’s disease; ChIP, chromatin-immunoprecipitation; Crohn’s Disease; EED, embryonic ectoderm development; EZH2, enhancer of zeste homolog 2; Epigenetics; FCS, fetal calf serum; FOXP3, forkhead domain-containing X-chromosome–encoded protein; H3K27me3, trimethylated histone H3 at lysine 27; IBD, inflammatory bowel disease; IL, interleukin; IPEX, immune dysregulation, polyendocrinopathy, enteropathy, X-linked; JAK, Janus kinase; LZ, leucine zipper; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; PLA, proximity ligation assay; PMA, phorbol 12-myristate 13-acetate; PRC2, polycomb repressive complex 2; Proinflammatory Cytokine; Regulatory T Cells; STAT, signal transducer and activator of transcription; SUZ12, suppressor of zeste; Th, T helper; Treg, regulatory T cell; WT, wild-type; co-IP, co-immunoprecipitation.
Figures





References
-
- Cho J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. - PubMed
-
- Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. - PubMed
-
- Isaacs K.L., Sartor R.B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992;103:1587–1595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

