Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission
- PMID: 30511960
- PMCID: PMC6355224
- DOI: 10.1172/JCI120606
Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission
Abstract
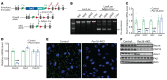
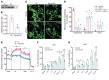
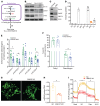
Peroxisomes perform essential functions in lipid metabolism, including fatty acid oxidation and plasmalogen synthesis. Here, we describe a role for peroxisomal lipid metabolism in mitochondrial dynamics in brown and beige adipocytes. Adipose tissue peroxisomal biogenesis was induced in response to cold exposure through activation of the thermogenic coregulator PRDM16. Adipose-specific knockout of the peroxisomal biogenesis factor Pex16 (Pex16-AKO) in mice impaired cold tolerance, decreased energy expenditure, and increased diet-induced obesity. Pex16 deficiency blocked cold-induced mitochondrial fission, decreased mitochondrial copy number, and caused mitochondrial dysfunction. Adipose-specific knockout of the peroxisomal β-oxidation enzyme acyl-CoA oxidase 1 (Acox1-AKO) was not sufficient to affect adiposity, thermogenesis, or mitochondrial copy number, but knockdown of the plasmalogen synthetic enzyme glyceronephosphate O-acyltransferase (GNPAT) recapitulated the effects of Pex16 inactivation on mitochondrial morphology and function. Plasmalogens are present in mitochondria and decreased with Pex16 inactivation. Dietary supplementation with plasmalogens increased mitochondrial copy number, improved mitochondrial function, and rescued thermogenesis in Pex16-AKO mice. These findings support a surprising interaction between peroxisomes and mitochondria regulating mitochondrial dynamics and thermogenesis.
Keywords: Adipose tissue; Cell Biology; Metabolism; Mitochondria.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL125838/HL/NHLBI NIH HHS/United States
- R01 DK115867/DK/NIDDK NIH HHS/United States
- K99 DK094874/DK/NIDDK NIH HHS/United States
- R03 DK109888/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- R01 DK107397/DK/NIDDK NIH HHS/United States
- T32 DK007120/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- I01 BX003415/BX/BLRD VA/United States
- R00 DK094874/DK/NIDDK NIH HHS/United States
- P30 AR057235/AR/NIAMS NIH HHS/United States
- R01 DK118333/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

