Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy With Hospitalization for Upper Gastrointestinal Tract Bleeding
- PMID: 30512099
- PMCID: PMC6404233
- DOI: 10.1001/jama.2018.17242
Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy With Hospitalization for Upper Gastrointestinal Tract Bleeding
Abstract
Importance: Anticoagulant choice and proton pump inhibitor (PPI) cotherapy could affect the risk of upper gastrointestinal tract bleeding, a frequent and potentially serious complication of oral anticoagulant treatment.
Objectives: To compare the incidence of hospitalization for upper gastrointestinal tract bleeding in patients using individual anticoagulants with and without PPI cotherapy, and to determine variation according to underlying gastrointestinal bleeding risk.
Design, setting, and participants: Retrospective cohort study in Medicare beneficiaries between January 1, 2011, and September 30, 2015.
Exposures: Apixaban, dabigatran, rivaroxaban, or warfarin with or without PPI cotherapy.
Main outcomes and measures: Hospitalizations for upper gastrointestinal tract bleeding: adjusted incidence and risk difference (RD) per 10 000 person-years of anticoagulant treatment, incidence rate ratios (IRRs).
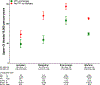
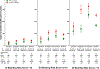
Results: There were 1 643 123 patients with 1 713 183 new episodes of oral anticoagulant treatment included in the cohort (mean [SD] age, 76.4 [2.4] years, 651 427 person-years of follow-up [56.1%] were for women, and the indication was atrial fibrillation for 870 330 person-years [74.9%]). During 754 389 treatment person-years without PPI cotherapy, the adjusted incidence of hospitalization for upper gastrointestinal tract bleeding (n = 7119) was 115 per 10 000 person-years (95% CI, 112-118). The incidence for rivaroxaban (n = 1278) was 144 per 10 000 person-years (95% CI, 136-152), which was significantly greater than the incidence of hospitalizations for apixaban (n = 279; 73 per 10 000 person-years; IRR, 1.97 [95% CI, 1.73-2.25]; RD, 70.9 [95% CI, 59.1-82.7]), dabigatran (n = 629; 120 per 10 000 person-years; IRR, 1.19 [95% CI, 1.08-1.32]; RD, 23.4 [95% CI, 10.6-36.2]), and warfarin (n = 4933; 113 per 10 000 person-years; IRR, 1.27 [95% CI, 1.19-1.35]; RD, 30.4 [95% CI, 20.3-40.6]). The incidence for apixaban was significantly lower than that for dabigatran (IRR, 0.61 [95% CI, 0.52-0.70]; RD, -47.5 [95% CI,-60.6 to -34.3]) and warfarin (IRR, 0.64 [95% CI, 0.57-0.73]; RD, -40.5 [95% CI, -50.0 to -31.0]). When anticoagulant treatment with PPI cotherapy (264 447 person-years; 76 per 10 000 person-years) was compared with treatment without PPI cotherapy, risk of upper gastrointestinal tract bleeding hospitalizations (n = 2245) was lower overall (IRR, 0.66 [95% CI, 0.62-0.69]) and for apixaban (IRR, 0.66 [95% CI, 0.52-0.85]; RD, -24 [95% CI, -38 to -11]), dabigatran (IRR, 0.49 [95% CI, 0.41-0.59]; RD, -61.1 [95% CI, -74.8 to -47.4]), rivaroxaban (IRR, 0.75 [95% CI, 0.68-0.84]; RD, -35.5 [95% CI, -48.6 to -22.4]), and warfarin (IRR, 0.65 [95% CI, 0.62-0.69]; RD, -39.3 [95% CI, -44.5 to -34.2]).
Conclusions and relevance: Among patients initiating oral anticoagulant treatment, incidence of hospitalization for upper gastrointestinal tract bleeding was the highest in patients prescribed rivaroxaban, and the lowest for patients prescribed apixaban. For each anticoagulant, the incidence of hospitalization for upper gastrointestinal tract bleeding was lower among patients who were receiving PPI cotherapy. These findings may inform assessment of risks and benefits when choosing anticoagulant agents.
Conflict of interest statement
Figures

Comment in
-
[Prevention of gastrointestinal bleeding by means of proton pump inhibitors].Internist (Berl). 2019 May;60(5):545-548. doi: 10.1007/s00108-019-0587-6. Internist (Berl). 2019. PMID: 30887069 German. No abstract available.
References
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. - PubMed
-
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. - PubMed
-
- Mazurek M, Lip GYH. Gastrointestinal Bleeding and Direct Oral Anticoagulants Amongst Patients With Atrial Fibrillation in the “Real World”. Gastroenterology. 2017;152(5):932–934. - PubMed
-
- Graham DJ, Reichman ME, Wernecke M, et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated With Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA internal medicine. 2016;176(11):1662–1671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

