Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: A randomised controlled trial
- PMID: 30513108
- PMCID: PMC6279020
- DOI: 10.1371/journal.pmed.1002706
Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: A randomised controlled trial
Abstract
Background: In sub-Saharan Africa, individuals infected with HIV who are severely immunocompromised have high mortality (about 10%) shortly after starting antiretroviral therapy (ART). This group also has the greatest risk of morbidity and mortality associated with immune reconstitution inflammatory syndrome (IRIS), a paradoxical response to successful ART. Integrase inhibitors lead to significantly more rapid declines in HIV viral load (VL) than all other ART classes. We hypothesised that intensifying standard triple-drug ART with the integrase inhibitor, raltegravir, would reduce HIV VL faster and hence reduce early mortality, although this strategy could also risk more IRIS events.
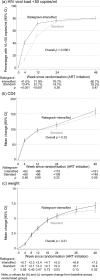
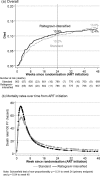
Methods and findings: In a 2×2×2 factorial open-label parallel-group trial, treatment-naive adults, adolescents, and children >5 years old infected with HIV, with cluster of differentiation 4 (CD4) <100 cells/mm3, from eight urban/peri-urban HIV clinics at regional hospitals in Kenya, Malawi, Uganda, and Zimbabwe were randomised 1:1 to initiate standard triple-drug ART, with or without 12-week raltegravir intensification, and followed for 48 weeks. The primary outcome was 24-week mortality, analysed by intention to treat. Of 2,356 individuals screened for eligibility, 1,805 were randomised between 18 June 2013 and 10 April 2015. Of the 1,805 participants, 961 (53.2%) were male, 72 (4.0%) were children/adolescents, median age was 36 years, CD4 count was 37 cells/mm3, and plasma viraemia was 249,770 copies/mL. Fifty-six participants (3.1%) were lost to follow-up at 48 weeks. By 24 weeks, 97/902 (10.9%) raltegravir-intensified ART versus 91/903 (10.2%) standard ART participants had died (adjusted hazard ratio [aHR] = 1.10 [95% CI 0.82-1.46], p = 0.53), with no evidence of interaction with other randomisations (pheterogeneity > 0.7) and despite significantly greater VL suppression with raltegravir-intensified ART at 4 weeks (343/836 [41.0%] versus 113/841 [13.4%] with standard ART, p < 0.001) and 12 weeks (567/789 [71.9%] versus 415/803 [51.7%] with standard ART, p < 0.001). Through 48 weeks, there was no evidence of differences in mortality (aHR = 0.98 [95% CI 0.76-1.28], p = 0.91); in serious (aHR = 0.99 [0.81-1.21], p = 0.88), grade-4 (aHR = 0.88 [0.71-1.09], p = 0.29), or ART-modifying (aHR = 0.90 [0.63-1.27], p = 0.54) adverse events (the latter occurring in 59 [6.5%] participants with raltegravir-intensified ART versus 66 [7.3%] with standard ART); in events judged compatible with IRIS (occurring in 89 [9.9%] participants with raltegravir-intensified ART versus 86 [9.5%] with standard ART, p = 0.79) or in hospitalisations (aHR = 0.94 [95% CI 0.76-1.17], p = 0.59). At 12 weeks, one and two raltegravir-intensified participants had predicted intermediate-level and high-level raltegravir resistance, respectively. At 48 weeks, the nucleoside reverse transcriptase inhibitor (NRTI) mutation K219E/Q (p = 0.004) and the non-nucleoside reverse transcriptase inhibitor (NNRTI) mutations K101E/P (p = 0.03) and P225H (p = 0.007) were less common in virus from participants with raltegravir-intensified ART, with weak evidence of less intermediate- or high-level resistance to tenofovir (p = 0.06), abacavir (p = 0.08), and rilpivirine (p = 0.07). Limitations of the study include limited clinical, radiological, and/or microbiological information for some participants, reflecting available services at the centres, and lack of baseline genotypes.
Conclusions: Although 12 weeks of raltegravir intensification was well tolerated and reduced HIV viraemia significantly faster than standard triple-drug ART during the time of greatest risk for early death, this strategy did not reduce mortality or clinical events in this group and is not warranted. There was no excess of IRIS-compatible events, suggesting that integrase inhibitors can be used safely as part of standard triple-drug first-line therapy in severely immunocompromised individuals.
Trial registration: ClinicalTrials.gov NCT01825031.
Trial registration: International Standard Randomised Controlled Trials Number ISRCTN 43622374.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: the institution of ASW has received funding from Janssen and Gilead Sciences for DSMB membership and lectures; JH has received funding for advisory board membership from Mylan Pharmaceuticals; all other authors have no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach (second edition) (http://www.who.int/hiv/pub/arv/arv-2016/en/). Geneva: 2016. - PubMed
-
- IeDea ART Cohort Collaborations, Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65(1):e8–16. 10.1097/QAI.0b013e3182a39979 ; PubMed Central PMCID: PMC3894575. - DOI - PMC - PubMed
-
- Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718 10.1371/journal.pmed.1001718 ; PubMed Central PMCID: PMC4159124. - DOI - PMC - PubMed
-
- Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, Giddy J, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010;24(14):2263–70. 10.1097/QAD.0b013e32833d45c5 ; PubMed Central PMCID: PMC2948209. - DOI - PMC - PubMed
-
- Walker AS, Prendergast AJ, Mugyenyi P, Munderi P, Hakim J, Kekitiinwa A, et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis. 2012;55(12):1707–18. 10.1093/cid/cis797 ; PubMed Central PMCID: PMC3501336. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

