Effects of acute hypoxia exposure with different durations on activation of Nrf2-ARE pathway in mouse skeletal muscle
- PMID: 30513114
- PMCID: PMC6279028
- DOI: 10.1371/journal.pone.0208474
Effects of acute hypoxia exposure with different durations on activation of Nrf2-ARE pathway in mouse skeletal muscle
Abstract
Background: Hypoxia training enhances the endurance capacity of athletes. This response may in part be attributed to the hypoxia-induced increase in antioxidant capacity in skeletal muscles. Nuclear factor erythroid 2-related factor 2 (Nrf2), a key transcription factor which regulates the expression of genes via binding to the antioxidant-response element (ARE) of these genes, plays a crucial role in stimulating the body's defense system and potentially responds to hypoxia. Meanwhile, hypoxia-inducible factor-1α (HIF-1α) is an important player in protecting cells from hypoxic stress. The purpose of this study was to investigate the effects of acute hypoxia exposure with different durations on the activation of Nrf2-ARE pathway and a possible regulatory role of HIF-1α in these responses.
Methods: C57BL/6J mice were allocated into the non-hypoxia 0-hour, 6-hour, 24-hour, and 48-hour hypoxic exposure (11.2% oxygen) groups. The quadriceps femoris was collected immediately after hypoxia. Further, to investigate the possible role of HIF-1α, C2C12 myoblasts with HIF-1α knockdown by small interfering RNA (siRNA) and the inducible HIF-1α transgenic mice were employed.
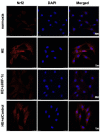
Results: The results showed that 48-hour hypoxia exposure up-regulated protein expression of Nrf2, Nrf2/ARE binding activity and the transcription of antioxidative genes containing ARE (Sod1 and others) in mouse skeletal muscle. Moreover, HIF-1α siRNA group of C2C12 myoblasts showed a remarkable inhibition of Nrf2 protein expression and nuclear accumulation in hypoxia exposure for 72 hours compared with that in siRNA-Control group of the cells. In addition, HIF-1α transgenic mice gave higher Nrf2 protein expression, Nrf2/ARE binding activity and expressions of Nrf2-mediated antioxidative genes in their skeletal muscle, compared with those in the wild-type mice.
Conclusions: The findings suggested that the acute hypoxia exposure could trigger the activation of Nrf2-ARE pathway, with longer duration associated with higher responses, and HIF-1α expression might be involved in promoting the Nrf2-mediated antioxidant responses in skeletal muscle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Ponsot E, Dufour SP, Zoll J, Doutrelau S, N'Guessan B, Geny B, et al. (2006) Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol (1985) 100: 1249–1257. 10.1152/japplphysiol.00361.2005 - DOI - PubMed
-
- Faiss R, Leger B, Vesin JM, Fournier PE, Eggel Y, Deriaz O, et al. (2013) Significant molecular and systemic adaptations after repeated sprint training in hypoxia. PLoS One 8: e56522 10.1371/journal.pone.0056522 - DOI - PMC - PubMed
-
- Bailey DM, Davies B, Young IS (2001) Intermittent hypoxic training: implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin Sci (Lond) 101: 465–475. - PubMed
-
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

