α-Melanocyte-Stimulating Hormone Attenuates Neovascularization by Inducing Nitric Oxide Deficiency via MC-Rs/PKA/NF-κB Signaling
- PMID: 30513637
- PMCID: PMC6321109
- DOI: 10.3390/ijms19123823
α-Melanocyte-Stimulating Hormone Attenuates Neovascularization by Inducing Nitric Oxide Deficiency via MC-Rs/PKA/NF-κB Signaling
Abstract
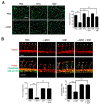
α-melanocyte-stimulating hormone (α-MSH) has been characterized as a novel angiogenesis inhibitor. The homeostasis of nitric oxide (NO) plays an important role in neovascularization. However, it remains unclear whether α-MSH mitigates angiogenesis through modulation of NO and its signaling pathway. The present study elucidated the function and mechanism of NO signaling in α-MSH-induced angiogenesis inhibition using cultured human umbilical vein endothelial cells (HUVECs), rat aorta rings, and transgenic zebrafish. By Griess reagent assay, it was found α-MSH dose-dependently reduced the NO release in HUVECs. Immunoblotting and immunofluorescence analysis revealed α-MSH potently suppressed endothelial and inducible nitric oxide synthase (eNOS/iNOS) expression, which was accompanied with inhibition of nuclear factor kappa B (NF-κB) activities. Excessive supply of NO donor l-arginine reversed the α-MSH-induced angiogenesis inhibition in vitro and in vivo. By using antibody neutralization and RNA interference, it was delineated that melanocortin-1 receptor (MC1-R) and melanocortin-2 receptor (MC2-R) participated in α-MSH-induced inhibition of NO production and NF-κB/eNOS/iNOS signaling. This was supported by pharmaceutical inhibition of protein kinase A (PKA), the downstream effector of MC-Rs signaling, using H89 abolished the α-MSH-mediated suppression of NO release and eNOS/iNOS protein level. Therefore, α-MSH exerts anti-angiogenic function by perturbing NO bioavailability and eNOS/iNOS expression in endothelial cells.
Keywords: HUVECs; melanocortin receptors (MC-Rs); nitric oxide (NO); α-melanocyte-stimulating hormone (α-MSH).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Brzoska T., Luger T.A., Maaser C., Abels C., Bohm M. α-melanocyte-stimulating hormone and related tripeptides: Biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr. Rev. 2008;29:581–602. doi: 10.1210/er.2007-0027. - DOI - PubMed
-
- Cyr N.E., Steger J.S., Toorie A.M., Yang J.Z., Stuart R., Nillni E.A. Central Sirt1 regulates body weight and energy expenditure along with the POMC-derived peptide α-MSH and the processing enzyme CPE production in diet-induced obese male rats. Endocrinology. 2015;156:961–974. doi: 10.1210/en.2014-1970. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

