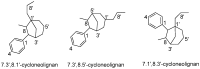
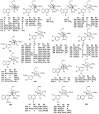
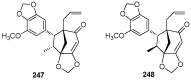
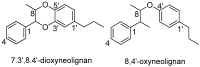
Chemical Structures of Lignans and Neolignans Isolated from Lauraceae
- PMID: 30513687
- PMCID: PMC6321345
- DOI: 10.3390/molecules23123164
Chemical Structures of Lignans and Neolignans Isolated from Lauraceae
Abstract
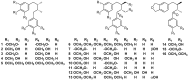
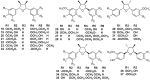
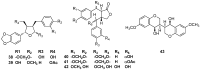
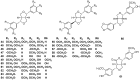
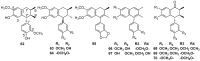
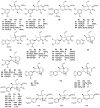
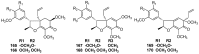
Lauraceae is a good source of lignans and neolignans, which are the most chemotaxonomic characteristics of many species of the family. This review describes 270 naturally occurring lignans and neolignans isolated from Lauraceae.
Keywords: Lauraceae; chemical components; chemical structures; lignans; neolignans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

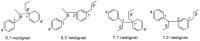





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

