Photo-Induced Depletion of Binding Sites in DNA-PAINT Microscopy
- PMID: 30513691
- PMCID: PMC6321339
- DOI: 10.3390/molecules23123165
Photo-Induced Depletion of Binding Sites in DNA-PAINT Microscopy
Abstract
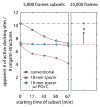
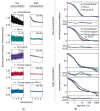
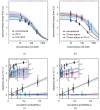
The limited photon budget of fluorescent dyes is the main limitation for localization precision in localization-based super-resolution microscopy. Points accumulation for imaging in nanoscale topography (PAINT)-based techniques use the reversible binding of fluorophores and can sample a single binding site multiple times, thus elegantly circumventing the photon budget limitation. With DNA-based PAINT (DNA-PAINT), resolutions down to a few nanometers have been reached on DNA-origami nanostructures. However, for long acquisition times, we find a photo-induced depletion of binding sites in DNA-PAINT microscopy that ultimately limits the quality of the rendered images. Here we systematically investigate the loss of binding sites in DNA-PAINT imaging and support the observations with measurements of DNA hybridization kinetics via surface-integrated fluorescence correlation spectroscopy (SI-FCS). We do not only show that the depletion of binding sites is clearly photo-induced, but also provide evidence that it is mainly caused by dye-induced generation of reactive oxygen species (ROS). We evaluate two possible strategies to reduce the depletion of binding sites: By addition of oxygen scavenging reagents, and by the positioning of the fluorescent dye at a larger distance from the binding site.
Keywords: DNA-PAINT; photo-induced DNA damage; reactive oxygen species; super-resolution microscopy; surface-integrated fluorescence correlation spectroscopy (SI-FCS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

