MicroRNA-Regulated Rickettsial Invasion into Host Endothelium via Fibroblast Growth Factor 2 and Its Receptor FGFR1
- PMID: 30513762
- PMCID: PMC6315532
- DOI: 10.3390/cells7120240
MicroRNA-Regulated Rickettsial Invasion into Host Endothelium via Fibroblast Growth Factor 2 and Its Receptor FGFR1
Abstract
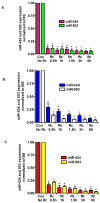
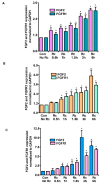
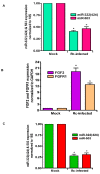
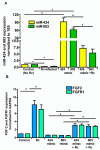
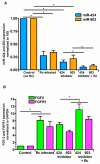
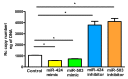
Microvascular endothelial cells (ECs) represent the primary target cells during human rickettsioses and respond to infection via the activation of immediate⁻early signaling cascades and the resultant induction of gene expression. As small noncoding RNAs dispersed throughout the genome, microRNAs (miRNAs) regulate gene expression post-transcriptionally to govern a wide range of biological processes. Based on our recent findings demonstrating the involvement of fibroblast growth factor receptor 1 (FGFR1) in facilitating rickettsial invasion into host cells and published reports suggesting miR-424 and miR-503 as regulators of FGF2/FGFR1, we measured the expression of miR-424 and miR-503 during R. conorii infection of human dermal microvascular endothelial cells (HMECs). Our results revealed a significant decrease in miR-424 and miR-503 expression in apparent correlation with increased expression of FGF2 and FGFR1. Considering the established phenomenon of endothelial heterogeneity and pulmonary and cerebral edema as the prominent pathogenic features of rickettsial infections, and significant pathogen burden in the lungs and brain in established mouse models of disease, we next quantified miR-424 and miR-503 expression in pulmonary and cerebral microvascular ECs. Again, R. conorii infection dramatically downregulated both miRNAs in these tissue-specific ECs as early as 30 min post-infection in correlation with higher FGF2/FGFR1 expression. Changes in the expression of both miRNAs and FGF2/FGFR1 were next confirmed in a mouse model of R. conorii infection. Furthermore, miR-424 overexpression via transfection of a mimic into host ECs reduced the expression of FGF2/FGFR1 and gave a corresponding decrease in R. conorii invasion, while an inhibitor of miR-424 had the expected opposite effect. Together, these findings implicate the rickettsial manipulation of host gene expression via regulatory miRNAs to ensure efficient cellular entry as the critical requirement to establish intracellular infection.
Keywords: FGF2; FGFR1; Rickettsia; endothelial cells; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sahni A., Narra H.P., Walker D.H., Sahni S.K. Endothelial activation and injury: The mechanisms of rickettsial vasculitis. In: Gavins F., Stokes K.Y., editors. Vascular Responses to Pathogens. Elsevier; Atlanta, GA, USA: 2016. pp. 111–122.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

